Abstract
Introduction
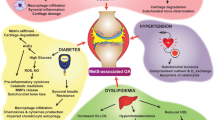
Today, not only the existence of an interrelation between obesity/adipositas and osteoarthritis (OA) but also the association of OA and diabetes mellitus (DM) are widely recognized. Nevertheless, shared influence factors facilitating OA development in DM patients still remain speculative up until now. To supplement the analysis of clinical data, appropriate in vitro models could help to identify shared pathogenetic pathways. Informative in vitro studies could later be complemented by in vivo data obtained from suitable animal models.
Materials and methods
Therefore, this detailed review of available literature was undertaken to discuss and compare the results of currently published in vitro studies focusing on the interrelation between OA, the metabolic syndrome and DM and to propose models to further study the molecular pathways.
Results
The survey of literature presented here supports the hypothesis that the pathogenesis of OA in DM is based on imbalanced molecular pathways with a putative crucial role of antiinflammatory cytokines such as IL-10.
Conclusion
Future development of versatile micro-scaled in vitro models such as combining DM and OA on chip could allow the identification of common pathogenetic pathways and might help to develop novel therapeutic strategies.




Similar content being viewed by others
Abbreviations
- ACL:
-
Anterior cruciate ligament
- AGE:
-
Advanced glycation end products
- BMI:
-
Body mass index
- BMP:
-
Bone morphogenetic protein
- CRP:
-
C-reactive protein
- 2D/3D:
-
Two/three dimensional
- DM:
-
Diabetes mellitus
- DMEM:
-
Dulbecco’s modified Eagles medium
- ECM:
-
Extracellular matrix
- ERK1/2:
-
Extracellular signal regulated kinase 1/2
- FBS:
-
Fetal bovine serum
- FFA:
-
Free fatty acids
- GAG:
-
Glycosaminoglycans
- 2-DG:
-
2-Deoxy-d-glucose
- GLU-1-3:
-
Glucose transporter 1-3
- HO-1:
-
Hemoxygenase-1
- IGF:
-
Insulin-like growth factor
- IGFR-1:
-
Insulin-like growth factor receptor 1
- IL:
-
Interleukin
- iNOS:
-
Inducible nitric oxide
- InsR:
-
Insulin receptor
- IR:
-
Insulin resistance
- LC3 II:
-
Lipid-modified microtubule-associated light chain 3 protein
- MMP:
-
Matrix metalloproteinase
- MSC:
-
Mesenchymal stromal cell
- NO:
-
Nitric oxide
- OA:
-
Osteoarthritis
- PCL:
-
Posterior cruciate ligament
- PGE2:
-
Prostaglandine E2
- PLGA:
-
Polylactic glycolic acid
- PPARα, γ:
-
Peroxisome proliferator-activated receptor α, γ
- PI3K:
-
Phosphoinositide 3-kinase
- RAGE:
-
Receptor of AGE
- ROS:
-
Reactive oxygen species
- RPMI medium:
-
Roswell Park Memorial Institute medium
- SMAD1/5/8:
-
Small body size/mothers against decapentaplegic 1/5/8
- STAT3:
-
Signal transducer and activator of transcription
- TNF:
-
Tumor necrosis factor
- T1DM:
-
Type 1 diabetes mellitus
- T2DM:
-
Type 2 diabetes mellitus
- VAS:
-
Visual analogue scale
- VEGF:
-
Vascular endothelial growth factor
- WOMAC:
-
Western Ontario and McMaster Universities Arthritis Index
References
Krasnokutsky S, Samuels J, Abramson SB. Osteoarthritis in 2007. Bull NYU Hosp Jt Dis. 2007;65(3):222–8.
Murphy NJ, Eyles JP, Hunter DJ. Hip osteoarthritis: etiopathogenesis and implications for management. Adv Ther. 2016;33(11):1921–46.
Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64(6):1697–707.
Das SK, Farooqi A. Osteoarthritis. Best Pract Res Clin Rheumatol. 2008;22(4):657–75.
Courties A, Gualillo O, Berenbaum F, Sellam J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthr Cartil. 2015;23(11):1955–65.
Martyniak K, Masternak MM. Changes in adipose tissue cellular composition during obesity and aging as a cause of metabolic dysregulation. Exp Gerontol. 2017;94:59–63.
Divella R, De Luca R, Abbate I, Naglieri E, Daniele A. Obesity and cancer: the role of adipose tissue and adipo-cytokines-induced chronic inflammation. J Cancer. 2016;7(15):2346–59.
Yates N, Teuner CM, Hunger M, Holle R, Stark R, Laxy M, et al. The economic burden of obesity in Germany: results from the population-based KORA studies. Obes Facts. 2016;9(6):397–409.
Chang SC, Yang WV. Hyperglycemia, tumorigenesis, and chronic inflammation. Crit Rev Oncol Hematol. 2016;108:146–53.
Petersen AM, Pedersen BK. The anti-inflammatory effect of exercise. J Appl Physiol. 2005;98(4):1154–62.
Louati K, Vidal C, Berenbaum F, Sellam J. Association between diabetes mellitus and osteoarthritis: systematic literature review and meta-analysis. RMD Open. 2015;1(1):e000077.
El Karib AO, Al-Ani B, Al-Hashem F, Dallak M, Bin-Jaliah I, El Gamal B, et al. Insulin and vanadium protect against osteoarthritis development secondary to diabetes mellitus in Rats. Arch Physiol Biochem. 2016;122(3):148–54.
Hopps E, Canino B, Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetol. 2011;48(3):183–9.
Sokolove J, Lepus CM. Role of inflammation in the pathogenesis of osteoarthritis: latest findings and interpretations. Ther Adv Musculoskelet Dis. 2013;5(2):77–94.
Courties A, Sellam J. Osteoarthritis and type 2 diabetes mellitus: What are the links? Diabetes Res Clin Pract. 2016;122:198–206.
Wang G. Raison d’etre of insulin resistance: the adjustable threshold hypothesis. J R Soc Interface. 2014;11(101):20140892.
Paniagua JA. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J Diabetes. 2016;7(19):483–514.
Schinner S, Scherbaum WA, Bornstein SR, Barthel A. Molecular mechanisms of insulin resistance. Diabet Med. 2005;22(6):674–82.
Ward CW, Lawrence MC. Ligand-induced activation of the insulin receptor: a multi-step process involving structural changes in both the ligand and the receptor. Bioessays. 2009;31(4):422–34.
Rosa SC, Rufino AT, Judas F, Tenreiro C, Lopes MC, Mendes AF. Expression and function of the insulin receptor in normal and osteoarthritic human chondrocytes: modulation of anabolic gene expression, glucose transport and GLUT-1 content by insulin. Osteoarthr Cartil. 2011;19(6):719–27.
Bessueille L, Fakhry M, Hamade E, Badran B, Magne D. Glucose stimulates chondrocyte differentiation of vascular smooth muscle cells and calcification: a possible role for IL-1beta. FEBS Lett. 2015;589(19 Pt B):2797–804.
Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55(Suppl 2):S9–15.
Malaguarnera R, Sacco A, Voci C, Pandini G, Vigneri R, Belfiore A. Proinsulin binds with high affinity the insulin receptor isoform A and predominantly activates the mitogenic pathway. Endocrinology. 2012;153(5):2152–63.
Negre-Salvayre A, Salvayre R, Auge N, Pamplona R, Portero-Otin M. Hyperglycemia and glycation in diabetic complications. Antioxid Redox Signal. 2009;11(12):3071–109.
Abe R, Yamagishi S. AGE-RAGE system and carcinogenesis. Curr Pharm Des. 2008;14(10):940–5.
Barry JC, Shakibakho S, Durrer C, Simtchouk S, Jawanda KK, Cheung ST, et al. Hyporesponsiveness to the anti-inflammatory action of interleukin-10 in type 2 diabetes. Sci Rep. 2016;6:21244.
Piva SR, Susko AM, Khoja SS, Josbeno DA, Fitzgerald GK, Toledo FG. Links between osteoarthritis and diabetes: implications for management from a physical activity perspective. Clin Geriatr Med. 2015;31(1):67–87.
de Lange-Brokaar BJ, Ioan-Facsinay A, Yusuf E, Kroon HM, Zuurmond AM, Stojanovic-Susulic V, et al. Evolution of synovitis in osteoarthritic knees and its association with clinical features. Osteoarthr Cartil. 2016;24(11):1867–74.
Ruschke K, Meier C, Ullah M, Krebs AC, Silberreis K, Kohl B, et al. Bone morphogenetic protein 2/SMAD signalling in human ligamentocytes of degenerated and aged anterior cruciate ligaments. Osteoarthr Cartil. 2016;24(10):1816–25.
Pauli C, Grogan SP, Patil S, Otsuki S, Hasegawa A, Koziol J, et al. Macroscopic and histopathologic analysis of human knee menisci in aging and osteoarthritis. Osteoarthr Cartil. 2011;19(9):1132–41.
Fernandes JC, Martel-Pelletier J, Pelletier J-P. The role of cytokines in osteoarthritis pathophysiology. Biorheology. 2002;39:237–46.
Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459.
Mabey T, Honsawek S. Cytokines as biochemical markers for knee osteoarthritis. World J Orthop. 2015;6(1):95–105.
Rojas-Ortega M, Cruz R, Vega-Lopez MA, Cabrera-Gonzalez M, Hernandez-Hernandez JM, Lavalle-Montalvo C, et al. Exercise modulates the expression of IL-1beta and IL-10 in the articular cartilage of normal and osteoarthritis-induced rats. Pathol Res Pract. 2015;211(6):435–43.
Jarvinen K, Vuolteenaho K, Nieminen R, Moilanen T, Knowles RG, Moilanen E. Selective iNOS inhibitor 1400W enhances anti-catabolic IL-10 and reduces destructive MMP-10 in OA cartilage. Survey of the effects of 1400W on inflammatory mediators produced by OA cartilage as detected by protein antibody array. Clin Exp Rheumatol. 2008;26(2):275–82.
Schulze-Tanzil G, Zreiqat H, Sabat R, Kohl B, Halder A, Muller RD, et al. Interleukin-10 and articular cartilage: experimental therapeutical approaches in cartilage disorders. Curr Gene Ther. 2009;9(4):306–15.
John T, Muller RD, Oberholzer A, Zreiqat H, Kohl B, Ertel W, et al. Interleukin-10 modulates pro-apoptotic effects of TNF-alpha in human articular chondrocytes in vitro. Cytokine. 2007;40(3):226–34.
Muller RD, John T, Kohl B, Oberholzer A, Gust T, Hostmann A, et al. IL-10 overexpression differentially affects cartilage matrix gene expression in response to TNF-alpha in human articular chondrocytes in vitro. Cytokine. 2008;44(3):377–85.
Imamura M, Ezquerro F, Marcon Alfieri F, Vilas Boas L, Tozetto-Mendoza TR, Chen J, et al. Serum levels of proinflammatory cytokines in painful knee osteoarthritis and sensitization. Int J Inflam. 2015;2015:329792.
Teunis T, Beekhuizen M, Van Osch GV, Schuurman AH, Creemers LB, van Minnen LP. Soluble mediators in posttraumatic wrist and primary knee osteoarthritis. Arch Bone Jt Surg. 2014;2(3):146–50.
Iannone F, De Bari C, Dell’Accio F, Covelli M, Cantatore FP, Patella V, et al. Interleukin-10 and interleukin-10 receptor in human osteoarthritic and healthy chondrocytes. Clin Exp Rheumatol. 2001;19(2):139–45.
Adams SB Jr, Nettles DL, Jones LC, Miller SD, Guyton GP, Schon LC. Inflammatory cytokines and cellular metabolites as synovial fluid biomarkers of posttraumatic ankle arthritis. Foot Ankle Int. 2014;35(12):1241–9.
Botha-Scheepers S, Watt I, Slagboom E, de Craen AJ, Meulenbelt I, Rosendaal FR, et al. Innate production of tumour necrosis factor alpha and interleukin 10 is associated with radiological progression of knee osteoarthritis. Ann Rheumatic Dis. 2008;67(8):1165–9.
Cattano NM, Driban JB, Balasubramanian E, Barbe MF, Amin M, Sitler MR. Biochemical comparison of osteoarthritic knees with and without effusion. BMC Musculoskelet Disord. 2011;12:273.
Hughes C, Sette A, Seed M, D’Acquisto F, Manzo A, Vincent TL, et al. Targeting of viral interleukin-10 with an antibody fragment specific to damaged arthritic cartilage improves its therapeutic potency. Arthritis Res Ther. 2014;16(4):R151.
Iannone F, Lapadula G. Obesity and inflammation–targets for OA therapy. Curr Drug Targets. 2010;11(5):586–98.
King LK, March L, Anandacoomarasamy A. Obesity and osteoarthritis. Indian J Med Res. 2013;138:185–93.
Livshits G, Zhai G, Hart DJ, Kato BS, Wang H, Williams FM, et al. Interleukin-6 is a significant predictor of radiographic knee osteoarthritis: the Chingford Study. Arthritis Rheum. 2009;60(7):2037–45.
Coggon D, Reading I, Croft P, McLaren M, Barrett D, Cooper C. Knee osteoarthritis and obesity. Int J Obese Relat Metab Disord. 2001;25(5):622–7.
Ioan-Facsinay A, Kloppenburg M. An emerging player in knee osteoarthritis: the infrapatellar fat pad. Arthritis Res Ther. 2013;15(6):225.
de Jong AJ, Klein-Wieringa IR, Andersen SN, Kwekkeboom JC, Herb-van Toorn L, de Lange-Brokaar BJE, et al. Lack of high BMI-related features in adipocytes and inflammatory cells in the infrapatellar fat pad (IFP). Arthritis Res Ther. 2017;19(1):186.
Skalska U, Kontny E. Adipose-derived mesenchymal stem cells from infrapatellar fat pad of patients with rheumatoid arthritis and osteoarthritis have comparable immunomodulatory properties. Autoimmunity. 2016;49(2):124–31.
Soleymaninejadian E, Pramanik K, Samadian E. Immunomodulatory properties of mesenchymal stem cells: cytokines and factors. Am J Reprod Immunol. 2012;67(1):1–8.
Najar M, Raicevic G, Crompot E, Fayyad-Kazan H, Bron D, Toungouz M, et al. The immunomodulatory potential of mesenchymal stromal cells: a story of a regulatory network. J Immunother. 2016;39(2):45–59.
Bao JP, Chen WP, Feng J, Hu PF, Shi ZL, Wu LD. Leptin plays a catabolic role on articular cartilage. Mol Biol Rep. 2010;37(7):3265–72.
Pearson MJ, Herndler-Brandstetter D, Tariq MA, Nicholson TA, Philp AM, Smith HL, et al. IL-6 secretion in osteoarthritis patients is mediated by chondrocyte-synovial fibroblast cross-talk and is enhanced by obesity. Sci Rep. 2017;7(1):3451.
Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395(6704):763–70.
Clockaerts S, Bastiaansen-Jenniskens YM, Feijt C, Verhaar JA, Somville J, De Clerck LS, et al. Peroxisome proliferator activated receptor alpha activation decreases inflammatory and destructive responses in osteoarthritic cartilage. Osteoarthr Cartil. 2011;19(7):895–902.
Philp AM, Collier RL, Grover LM, Davis ET, Jones SW. Resistin promotes the abnormal type I collagen phenotype of subchondral bone in obese patients with end stage hip osteoarthritis. Sci Rep. 2017;7(1):4042.
Santangelo KS, Radakovich LB, Fouts J, Foster MT. Pathophysiology of obesity on knee joint homeostasis: contributions of the infrapatellar fat pad. Horm Mol Biol Clin Investig. 2016;26(2):97–108.
Jung SH, Park HS, Kim KS, Choi WH, Ahn CW, Kim BT, et al. Effect of weight loss on some serum cytokines in human obesity: increase in IL-10 after weight loss. J Nutr Biochem. 2008;19(6):371–5.
Puenpatom RA, Victor TW. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: an analysis of NHANES III data. Postgrad Med. 2009;121(6):9–20.
Zhuo Q, Yang W, Chen J, Wang Y. Metabolic syndrome meets osteoarthritis. Nature Rev Rheumat. 2012;8(12):729–37.
Le Clanche S, Bonnefont-Rousselot D, Sari-Ali E, Rannou F, Borderie D. Inter-relations between osteoarthritis and metabolic syndrome: a common link? Biochimie. 2016;121:238–52.
Courties A, Sellam J, Berenbaum F. Metabolic syndrome-associated osteoarthritis. Curr Opin Rheumatol. 2017;29(2):214–22.
Niu J, Clancy M, Aliabadi P, Vasan R, Felson DT. Metabolic syndrome, its components, and knee osteoarthritis: the Framingham osteoarthritis study. Arthritis Rheum. 2017;69(6):1194–203.
Strand MP, Neogi T, Niu J, Felson DT, Haugen IK. No association between metabolic syndrome and radiographic hand osteoarthritis: data from the Framingham study. Arthritis Care Res. 2017. https://doi.org/10.1002/acr.23288.
Bray GA, Bellanger T. Epidemiology, trends, and morbidities of obesity and the metabolic syndrome. Endocrine. 2006;29(1):109–17.
Abourazzak F, Talbi S, Lazrak F, Azzouzi H, Aradoini N, Keita S, et al. Does metabolic syndrome or its individual components affect pain and function in knee osteoarthritis women? Curr Rheumatol Rev. 2015;11(1):8–14.
Miller RE, Miller RJ, Malfait AM. Osteoarthritis joint pain: the cytokine connection. Cytokine. 2014;70(2):185–93.
Ailixiding M, Aibibula Z, Iwata M, Piao J, Hara Y, Koga D, et al. Pivotal role of Sirt6 in the crosstalk among ageing, metabolic syndrome and osteoarthritis. Biochem Biophys Res Comm. 2015;466(3):319–26.
Kolaczynski JW, Nyce MR, Considine RV, Boden G, Nolan JJ, Henry R, et al. Acute and chronic effects of insulin on leptin production in humans: studies in vivo and in vitro. Diabetes. 1996;45(5):699–701.
Nagaev I, Bokarewa M, Tarkowski A, Smith U. Human resistin is a systemic immune-derived proinflammatory cytokine targeting both leukocytes and adipocytes. PLoS One. 2006;1:e31.
Rufino AT, Rosa SC, Judas F, Mobasheri A, Lopes MC, Mendes AF. Expression and function of K(ATP) channels in normal and osteoarthritic human chondrocytes: possible role in glucose sensing. J Cell Biochem. 2013;114(8):1879–89.
Davies-Tuck ML, Wang Y, Wluka AE, Berry PA, Giles GG, English DR, et al. Increased fasting serum glucose concentration is associated with adverse knee structural changes in adults with no knee symptoms and diabetes. Maturitas. 2012;72(4):373–8.
Marik W, Nemec SF, Zbyn S, Zalaudek M, Ludvik B, Riegler G, et al. Changes in cartilage and tendon composition of patients with type I diabetes mellitus: identification by quantitative sodium magnetic resonance imaging at 7 T. Invest Radiol. 2016;51(4):266–72.
van Meegeren ME, Roosendaal G, Jansen NW, Wenting MJ, van Wesel AC, van Roon JA, et al. IL-4 alone and in combination with IL-10 protects against blood-induced cartilage damage. Osteoarthr Cartil. 2012;20(7):764–72.
Chen YH, Hsieh SC, Chen WY, Li KJ, Wu CH, Wu PC, et al. Spontaneous resolution of acute gouty arthritis is associated with rapid induction of the anti-inflammatory factors TGFbeta1, IL-10 and soluble TNF receptors and the intracellular cytokine negative regulators CIS and SOCS3. Ann Rheum Dis. 2011;70(9):1655–63.
Moo V, Sieper J, Herzog V, Muller BM. Regulation of expression of cytokines and growth factors in osteoarthritic cartilage explants. Clin Rheumatol. 2001;20(5):353–8.
Huang TL, Hsu HC, Yang KC, Lin FH. Hyaluronan up-regulates IL-10 expression in fibroblast-like synoviocytes from patients with tibia plateau fracture. J Orthop Res. 2011;29(4):495–500.
Mrosewski I, Jork N, Gorte K, Conrad C, Wiegand E, Kohl B, et al. Regulation of osteoarthritis-associated key mediators by TNFalpha and IL-10: effects of IL-10 overexpression in human synovial fibroblasts and a synovial cell line. Cell Tissue Res. 2014;357(1):207–23.
Wyatt LA, Moreton BJ, Mapp PI, Wilson D, Hill R, Ferguson E, et al. Histopathological subgroups in knee osteoarthritis. Osteoarthr Cartil. 2017;25(1):14–22.
Oyama K, Taniguchi J, Goto R, Matsui K. Remitting seronegative symmetrical synovitis with pitting edema syndrome in individuals with type 2 diabetes mellitus or impaired glucose tolerance. Diabetes Res Clin Pract. 2015;110(1):e5–8.
Jung YK, Kim GW, Park HR, Lee EJ, Choi JY, Beier F, et al. Role of interleukin-10 in endochondral bone formation in mice: anabolic effect via the bone morphogenetic protein/Smad pathway. Arthritis Rheum. 2013;65(12):3153–64.
Guillen M, Megias J, Gomar F, Alcaraz M. Haem oxygenase-1 regulates catabolic and anabolic processes in osteoarthritic chondrocytes. J Pathol. 2008;214(4):515–22.
Gasparini G, De Gori M, Paonessa F, Chiefari E, Brunetti A, Galasso O. Functional relationship between high mobility group A1 (HMGA1) protein and insulin-like growth factor-binding protein 3 (IGFBP-3) in human chondrocytes. Arthritis Res Ther. 2012;14(5):R207.
Jagielski M, Wolf J, Marzahn U, Volker A, Lemke M, Meier C, et al. The influence of IL-10 and TNFalpha on chondrogenesis of human mesenchymal stromal cells in three-dimensional cultures. Int J Mol Sci. 2014;15(9):15821–44.
Toh WS, Lai RC, Po Hui JH, Lim SK. MSC exosome as a cell-free MSC therapy for cartilage regeneration: implications for osteoarthritis treatment. Semin Cell Dev Biol. 2017;67:56–64.
Afizah H, Hui JH. Mesenchymal stem cell therapy for osteoarthritis. J Clin Orthop Trauma. 2016;7(3):177–82.
Cui GH, Wang YY, Li CJ, Shi CH, Wang WS. Efficacy of mesenchymal stem cells in treating patients with osteoarthritis of the knee: a meta-analysis. Exp Ther Med. 2016;12(5):3390–400.
Booth FW, Roberts CK, Laye MJ. Lack of exercise is a major cause of chronic diseases. Compr Physiol. 2012;2(2):1143–211.
Issa RI, Griffin TM. Pathobiology of obesity and osteoarthritis: integrating biomechanics and inflammation. Pathobiol Aging Age Relat Dis. 2012;2(2012).
Helmark IC, Mikkelsen UR, Borglum J, Rothe A, Petersen MC, Andersen O, et al. Exercise increases interleukin-10 levels both intraarticularly and peri-synovially in patients with knee osteoarthritis: a randomized controlled trial. Arthritis Res Ther. 2010;12(4):R126.
Chen YJ, Chan DC, Lan KC, Wang CC, Chen CM, Chao SC, et al. PPARgamma is involved in the hyperglycemia-induced inflammatory responses and collagen degradation in human chondrocytes and diabetic mouse cartilages. J Orthop Res. 2015;33(3):373–81.
Touvra AM, Volaklis KA, Spassis AT, Zois CE, Douda HD, Kotsa K, et al. Combined strength and aerobic training increases transforming growth factor-beta1 in patients with type 2 diabetes. Hormones. 2011;10(2):125–30.
Oberbach A, Tonjes A, Kloting N, Fasshauer M, Kratzsch J, Busse MW, et al. Effect of a 4 week physical training program on plasma concentrations of inflammatory markers in patients with abnormal glucose tolerance. Eur J Endocrinol. 2006;154(4):577–85.
Liu Y, Liu SX, Cai Y, Xie KL, Zhang WL, Zheng F. Effects of combined aerobic and resistance training on the glycolipid metabolism and inflammation levels in type 2 diabetes mellitus. J Phys Ther Sci. 2015;27(7):2365–71.
Chen YW, Chiu CC, Hsieh PL, Hung CH, Wang JJ. Treadmill training combined with insulin suppresses diabetic nerve pain and cytokines in rat sciatic nerve. Anesth Analg. 2015;121(1):239–46.
Kadoglou NP, Iliadis F, Angelopoulou N, Perrea D, Ampatzidis G, Liapis CD, et al. The anti-inflammatory effects of exercise training in patients with type 2 diabetes mellitus. Eur J Cardiovasc Prev Rehabil. 2007;14(6):837–43.
Yaghini N, Mahmoodi M, Asadikaram GR, Hassanshahi GH, Khoramdelazad H, Kazemi Arababadi M. Serum levels of interleukin 10 (IL-10) in patients with type 2 diabetes. Iran Red Crescent Med J. 2011;13(10):752.
Hong EG, Ko HJ, Cho YR, Kim HJ, Ma Z, Yu TY, et al. Interleukin-10 prevents diet-induced insulin resistance by attenuating macrophage and cytokine response in skeletal muscle. Diabetes. 2009;58(11):2525–35.
Madeshiya AK, Singh S, Dwivedi S, Konwar R, Natu SM, Ghatak A. Association of IL-10 gene (-1082A > G, -819C > T and -592C > A) polymorphism and its serum level with metabolic syndrome of north Indian subjects. J Genet. 2017;96(1):53–64.
Balasa B, La Cava A, Van Gunst K, Mocnik L, Balakrishna D, Nguyen N, et al. A mechanism for IL-10-mediated diabetes in the nonobese diabetic (NOD) mouse: ICAM-1 deficiency blocks accelerated diabetes. J Immunol. 2000;165(12):7330–7.
Herder C, Carstensen M, Ouwens DM. Anti-inflammatory cytokines and risk of type 2 diabetes. Diabetes Obes Metab. 2013;15(Suppl 3):39–50.
Hardin JA, Cobelli N, Santambrogio L. Consequences of metabolic and oxidative modifications of cartilage tissue. Nat Rev Rheumatol. 2015;11(9):521–9.
DeGroot J, Verzijl N, Jacobs KM, Budde M, Bank RA, Bijlsma JW, et al. Accumulation of advanced glycation endproducts reduces chondrocyte-mediated extracellular matrix turnover in human articular cartilage. Osteoarthr Cartil. 2001;9(8):720–6.
Chen YJ, Sheu ML, Tsai KS, Yang RS, Liu SH. Advanced glycation end products induce peroxisome proliferator-activated receptor gamma down-regulation-related inflammatory signals in human chondrocytes via Toll-like receptor-4 and receptor for advanced glycation end products. PLoS One. 2013;8(6):e66611.
Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J Bone Mineral Res. 2005;20(9):1647–58.
Chen YJ, Chan DC, Chiang CK, Wang CC, Yang TH, Lan KC, et al. Advanced glycation end-products induced VEGF production and inflammatory responses in human synoviocytes via RAGE-NF-kappaB pathway activation. J Orthop Res. 2016;34(5):791–800.
Ma C, Zhang Y, Li YQ, Chen C, Cai W, Zeng YL. The role of PPARgamma in advanced glycation end products-induced inflammatory response in human chondrocytes. PLoS One. 2015;10(5):e0125776.
Han Z, Liu Q, Sun C, Li Y. The interaction between obesity and RAGE polymorphisms on the risk of knee osteoarthritis in Chinese population. Cell Physiol Biochem. 2012;30(4):898–904.
Rosa SC, Goncalves J, Judas F, Mobasheri A, Lopes C, Mendes AF. Impaired glucose transporter-1 degradation and increased glucose transport and oxidative stress in response to high glucose in chondrocytes from osteoarthritic versus normal human cartilage. Arthritis Res Ther. 2009;11(3):R80.
Mobasheri A. Glucose: an energy currency and structural precursor in articular cartilage and bone with emerging roles as an extracellular signaling molecule and metabolic regulator. Front Endocrinol. 2012;3:153.
Laiguillon MC, Courties A, Houard X, Auclair M, Sautet A, Capeau J, et al. Characterization of diabetic osteoarthritic cartilage and role of high glucose environment on chondrocyte activation: toward pathophysiological delineation of diabetes mellitus-related osteoarthritis. Osteoarthr Cartil. 2015;23(9):1513–22.
Shikhman AR, Brinson DC, Lotz MK. Distinct pathways regulate facilitated glucose transport in human articular chondrocytes during anabolic and catabolic responses. J Physiol Endocrinol Metab. 2004;286(6):E980–E5.
Laiguillon MC, Houard X, Bougault C, Gosset M, Nourissat G, Sautet A, et al. Expression and function of visfatin (Nampt), an adipokine-enzyme involved in inflammatory pathways of osteoarthritis. Arthritis Res Ther. 2014;16(1):R38.
Altman R, Asch E, Bloch D, Bole G, Borenstein D, Brandt K, et al. Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum. 1986;29(8):1039–49.
Moo EK, Osman NA, Pingguan-Murphy B. The metabolic dynamics of cartilage explants over a long-term culture period. Clinics. 2011;66(8):1431–6.
Leyh M, Seitz A, Durselen L, Springorum HR, Angele P, Ignatius A, et al. Osteoarthritic cartilage explants affect extracellular matrix production and composition in cocultured bone marrow-derived mesenchymal stem cells and articular chondrocytes. Stem Cell Res Ther. 2014;5(3):77.
Ribeiro M, Lopez de Figueroa P, Blanco FJ, Mendes AF, Carames B. Insulin decreases autophagy and leads to cartilage degradation. Osteoarthr Cartil. 2016;24(4):731–9.
Badendick J, Godkin O, Kohl B, Meier C, Jagielski M, Huang Z, et al. Macroscopical, histological, and in vitro characterization of nonosteoarthritic versus osteoarthritic hip joint cartilage. Clin Med Insights Arthritis Musculoskelet Disord. 2016;9:65–74.
Mankin HJ, Dorfman H, Lippiello L, Zarins A. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Jt Surg Am. 1971;53(3):523–37.
Outerbridge RE. The etiology of chondromalacia patellae. J Bone Jt Surg Br. 1961;43-B:752–7.
Li YM, Schilling T, Benisch P, Zeck S, Meissner-Weigl J, Schneider D, et al. Effects of high glucose on mesenchymal stem cell proliferation and differentiation. Biochem Biophys Res Comm. 2007;363(1):209–15.
Fahmi H, Martel-Pelletier J, Pelletier JP, Kapoor M. Peroxisome proliferator-activated receptor gamma in osteoarthritis. Modern Rheumatol. 2011;21(1):1–9.
McNulty AL, Stabler TV, Vail TP, McDaniel GE, Kraus VB. Dehydroascorbate transport in human chondrocytes is regulated by hypoxia and is a physiologically relevant source of ascorbic acid in the joint. Arthritis Rheum. 2005;52(9):2676–85.
Hiraiwa H, Sakai T, Mitsuyama H, Hamada T, Yamamoto R, Omachi T, et al. Inflammatory effect of advanced glycation end products on human meniscal cells from osteoarthritic knees. Inflam Res. 2011;60(11):1039–48.
Tsai CH, Chiang YC, Chen HT, Huang PH, Hsu HC, Tang CH. High glucose induces vascular endothelial growth factor production in human synovial fibroblasts through reactive oxygen species generation. Biochim Biophys Acta. 2013;1830(3):2649–58.
Wang Y, Lou S. Direct protective effect of interleukin-10 on articular chondrocytes in vitro. Chinese Med J. 2001;114(7):723–5.
Sweeney C, Mackintosh D, Mason RM. UDP-sugar metabolism in Swarm rat chondrosarcoma chondrocytes. Biochem J. 1993;290(Pt 2):563–70.
Lee GM, Tioran ME, Jansen M, Graff RD, Kelley SS, Lin P. Development of selective tolerance to interleukin-1beta by human chondrocytes in vitro. J Cellular Physiol. 2002;192(1):113–24.
Lee RB, Urban JP. Evidence for a negative Pasteur effect in articular cartilage. Biochem J. 1997;321(Pt 1):95–102.
Tsai TL, Manner PA, Li WJ. Regulation of mesenchymal stem cell chondrogenesis by glucose through protein kinase C/transforming growth factor signaling. Osteoarthr Cartil. 2013;21(2):368–76.
Aguiari P, Leo S, Zavan B, Vindigni V, Rimessi A, Bianchi K, et al. High glucose induces adipogenic differentiation of muscle-derived stem cells. Proc Natl Acad Sci USA. 2008;105(4):1226–31.
Cramer C, Freisinger E, Jones RK, Slakey DP, Dupin CL, Newsome ER, et al. Persistent high glucose concentrations alter the regenerative potential of mesenchymal stem cells. Stem Cells Dev. 2010;19(12):1875–84.
Lo T, Ho JH, Yang MH, Lee OK. Glucose reduction prevents replicative senescence and increases mitochondrial respiration in human mesenchymal stem cells. Cell Transplant. 2011;20(6):813–25.
Li Y, Wang Y, Chubinskaya S, Schoeberl B, Florine E, Kopesky P, et al. Effects of insulin-like growth factor-1 and dexamethasone on cytokine-challenged cartilage: relevance to post-traumatic osteoarthritis. Osteoarthr Cartil. 2015;23(2):266–74.
Mueller MB, Blunk T, Appel B, Maschke A, Goepferich A, Zellner J, et al. Insulin is essential for in vitro chondrogenesis of mesenchymal progenitor cells and influences chondrogenesis in a dose-dependent manner. Int Orthop. 2013;37(1):153–8.
Longobardi L, Granero-Molto F, O’Rear L, Myers TJ, Li T, Kregor PJ, et al. Subcellular localization of IRS-1 in IGF-I-mediated chondrogenic proliferation, differentiation and hypertrophy of bone marrow mesenchymal stem cells. Growth Factors. 2009;27(5):309–20.
Longobardi L, O’Rear L, Aakula S, Johnstone B, Shimer K, Chytil A, et al. Effect of IGF-I in the chondrogenesis of bone marrow mesenchymal stem cells in the presence or absence of TGF-beta signaling. J Bone Miner Res. 2006;21(4):626–36.
Ashraf S, Cha BH, Kim JS, Ahn J, Han I, Park H, et al. Regulation of senescence associated signaling mechanisms in chondrocytes for cartilage tissue regeneration. Osteoarthr Cartil. 2016;24(2):196–205.
Nanda HS, Chen S, Zhang Q, Kawazoe N, Chen G. Collagen scaffolds with controlled insulin release and controlled pore structure for cartilage tissue engineering. Biomed Res Int. 2014;2014:623805.
Askari A, Ehrampoush E, Homayounfar R, Bahramali E, Farjam M. Serum insulin in pathogenesis and treatment of osteoarthritis. Med Hypotheses. 2017;99:45–6.
Akyol S, Comertoglu I, Firat R, Cakmak O, Yukselten Y, Erden G, et al. Effect of insulin on the mRNA expression of procollagen N-proteinases in chondrosarcoma OUMS-27 cells. Oncol Lett. 2015;10(2):1091–6.
Cakmak O, Comertoglu I, Firat R, Erdemli HK, Kursunlu SF, Akyol S, et al. The investigation of ADAMTS16 in insulin-induced human chondrosarcoma cells. Cancer Biother Radiopharm. 2015;30(6):255–60.
Chen YY, Silva PN, Syed AM, Sindhwani S, Rocheleau JV, Chan WC. Clarifying intact 3D tissues on a microfluidic chip for high-throughput structural analysis. Proc Natl Acad Sci USA. 2016;113(52):14915–20.
Zambon A, Zoso A, Gagliano O, Magrofuoco E, Fadini GP, Avogaro A, et al. High temporal resolution detection of patient-specific glucose uptake from human ex vivo adipose tissue on-chip. Analyt Chem. 2015;87(13):6535–43.
Liu XF, Yu JQ, Dalan R, Liu AQ, Luo KQ. Biological factors in plasma from diabetes mellitus patients enhance hyperglycaemia and pulsatile shear stress-induced endothelial cell apoptosis. Integr Biol. 2014;6(5):511–22.
Liu Y, Chen C, Summers S, Medawala W, Spence DM. C-peptide and zinc delivery to erythrocytes requires the presence of albumin: implications in diabetes explored with a 3D-printed fluidic device. Integr Biol. 2015;7(5):534–43.
Acknowledgements
The authors are grateful for financial support by the Kerscher foundation. They are also grateful for the support of Mr. Benjamin Kohl, Carola Meier and Jessica Badendick (Dep. of Trauma and Reconstructive Surgery, CBF, Charité-Berlin).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Jason J. McDougall.
Silke Schwarz and Ingo Mrosewski contributed equally to this work.
Rights and permissions
About this article
Cite this article
Schwarz, S., Mrosewski, I., Silawal, S. et al. The interrelation of osteoarthritis and diabetes mellitus: considering the potential role of interleukin-10 and in vitro models for further analysis. Inflamm. Res. 67, 285–300 (2018). https://doi.org/10.1007/s00011-017-1121-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1121-8