Abstract
Objectives
We have previously demonstrated that downregulation of the MyD88/TAK1-dependent signaling pathway associated with increased CYP4A1 expression and 20-HETE formation participates in the protective effect of N-(20-hydroxyeicosa-5[Z],14[Z]-dienoyl)glycine (5,14-HEDGE), a 20-HETE mimetic, against vascular hyporeactivity, hypotension, tachycardia, inflammation, and mortality in a rodent model of septic shock. The aim of this study was to determine whether increased renal and cardiovascular expression of PPARα/β/γ and RXRα associated with decreased expression and/or activity of AP-1 and importin-α3 participates in the protective effect of 5,14-HEDGE in response to systemic administration of lipopolysaccharide (LPS).
Methods
Conscious male Wistar rats received saline (4 ml/kg) or LPS (10 mg/kg) at time 0. Blood pressure and heart rate were measured using a tail-cuff device. Separate groups of LPS-treated rats were given 5,14-HEDGE (30 mg/kg) 1 h after injection of saline or LPS. The rats were killed 4 h after saline or LPS administration and the kidney, heart, thoracic aorta, and superior mesenteric artery were collected for measurement of protein expression.
Results
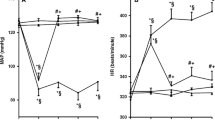
Blood pressure fell by 33 mmHg and heart rate rose by 72 beats/min at 4 h after LPS administration. In LPS-treated rats, tissue protein expressions of cytosolic/nuclear PPARα/β/γ and nuclear RXRα, in addition to nuclear translocation of PPARα/β/γ proteins, were decreased, while cytosolic/nuclear AP-1 subunit c-jun/phosphorylated c-jun and importin-α3 protein expression as well as their nuclear translocation were increased. The LPS-induced changes were prevented by 5,14-HEDGE.
Conclusions
The results suggest that an increase in the expression of PPARα/β/γ and RXRα as well as a decrease in AP-1 and importin-α3 expression/activity participates in the protective effect of 5,14-HEDGE against hypotension, tachycardia, and inflammation during endotoxemia and thus have a beneficial effect in septic shock treatment.















Similar content being viewed by others
Abbreviations
- 15d-PGJ2 :
-
15-Deoxy-Δ12,14-prostaglandin J2
- 20-HETE:
-
20-Hydroxyeicosatetraenoic acid
- 5,14-HEDGE:
-
N-(20-hydroxyeicosa-5[Z],14[Z]-dienoyl)glycine
- AP:
-
Activator protein
- BSA:
-
Bovine serum albumin
- CD:
-
Cluster of differentiation
- COX:
-
Cyclooxygenase
- Crm:
-
Chromosome region maintenance
- CYP:
-
Cyctochrome P450
- DNA:
-
Deoxyribonucleic acid
- ERK:
-
Extracellular signal-regulated kinase
- HR:
-
Heart rate
- Ig:
-
Immunoglobulin
- IKK:
-
IκB kinase
- IκB:
-
Inhibitor of κB
- IL:
-
Interleukin
- i.p.:
-
Intraperitoneally
- IL:
-
Interleukin
- IRAK:
-
Interleukin-1 receptor-associated kinase
- iNOS:
-
Inducible nitric oxide synthase
- LPS:
-
Lipopolysaccharide
- MAP:
-
Mean arterial pressure
- MAPK:
-
Mitogen-activated protein kinase
- MD:
-
Myeloid differentiation protein
- MEK:
-
Mitogen-activated protein kinase kinase
- MKK:
-
Mitogen-activated protein kinase kinase
- miRNA:
-
Microribonucleic acid
- MyD88:
-
Myeloid differentiation factor 88
- NADPH:
-
Nicotinamide adenine dinucleotide phosphate
- NES:
-
Nuclear export signals
- NF-κB:
-
Nuclear factor-κB
- NFAT:
-
Nuclear factor of activated T cells
- NLS:
-
Nuclear localization signals
- NO:
-
Nitric oxide
- PG:
-
Prostaglandin
- PPAR:
-
Peroxisome proliferator-activated receptor
- PPREs:
-
Peroxisome proliferator-activated receptor response elements
- RXR:
-
Retinoid X receptor
- s.c.:
-
Subcutaneously
- SEM:
-
Standard error of means
- STAT:
-
Signal transducer and activator of transcription
- TAK:
-
Transforming growth factor-activated kinase
- TIRAP:
-
Toll-interleukin-1 receptor domain containing adaptor protein
- TLR:
-
Toll-like receptor
- TNF:
-
Tumor necrosis factor
- TRAF:
-
TNF receptor-associated factor
References
Elshenawy OH, Anwar-Mohamed A, El-Kadi AO. 20-Hydroxyeicosatetraenoic acid is a potential therapeutic target in cardiovascular diseases. Curr Drug Metab. 2013;14:706–19.
Fan F, Muroya Y, Roman RJ. Cytochrome P450 eicosanoids in hypertension and renal disease. Curr Opin Nephrol Hypertens. 2015;24:37–46.
Roman RJ. P-450 metabolites of arachidonic acid in the control of cardiovascular function. Physiol Rev. 2002;82:131–85.
Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK, Sahan-Firat S, Schunck WH, Falck JR, Malik KU. A novel treatment strategy for sepsis and septic shock based on the interactions between prostanoids, nitric oxide, and 20-hydroxyeicosatetraenoic acid. Antiinflamm Antiallergy Agents Med Chem. 2012;11:121–50.
Wu CC, Gupta T, Garcia V, Ding Y, Schwartzman ML. 20-HETE and blood pressure regulation: clinical implications. Cardiol Rev. 2014;22:1–12.
Escalante B, Omata K, Sessa W, Lee SG, Falck JR, Schwartzman ML. 20-hydroxyeicosatetraenoic acid is an endothelium-dependent vasoconstrictor in rabbit arteries. Eur J Pharmacol. 1993;235:1–7.
Randriamboavonjy V, Busse R, Fleming I. 20-HETE-induced contraction of small coronary arteries depends on the activation of Rho-kinase. Hypertension. 2003;41:801–6.
Zou AP, Fleming JT, Falck JR, Jacobs ER, Gebremedhin D, Harder DR, Roman RJ. 20-HETE is an endogenous inhibitor of the large-conductance Ca(2+)-activated K+ channel in renal arterioles. Am J Physiol. 1996;270:R228–37.
Carroll MA, Capparelli MF, Doumand AB, Cheng MK, Jiang H, McGiff JC. Renal vasoactive eicosanoids: interactions between cytochrome P450 and cyclooxygenase metabolites during salt depletion. Am J Hypertens. 2001;14:159A.
Pratt PF, Falck JR, Reddy KM, Kurian JB, Campbell WB. 20-HETE relaxes bovine coronary arteries through the release of prostacyclin. Hypertension. 1998;31:237–41.
Cheng J, Wu CC, Gotlinger KH, Zhang F, Falck JR, Narsimhaswamy D, Schwartzman ML. 20-hydroxy-5,8,11,14-eicosatetraenoic acid mediates endothelial dysfunction via IkappaB kinase-dependent endothelial nitric-oxide synthase uncoupling. J Pharmacol Exp Ther. 2010;332:57–65.
Ishizuka T, Cheng J, Singh H, Vitto MD, Manthati VL, Falck JR, Laniado-Schwartzman M. 20-Hydroxyeicosatetraenoic acid stimulates nuclear factor-κB activation and the production of inflammatory cytokines in human endothelial cells. J Pharmacol Exp Ther. 2008;324:103–10.
Toth P, Csiszar A, Sosnowska D, Tucsek Z, Cseplo P, Springo Z, Tarantini S, Sonntag WE, Ungvari Z, Koller A. Treatment with the cytochrome P450 ω-hydroxylase inhibitor HET0016 attenuates cerebrovascular inflammation, oxidative stress and improves vasomotor function in spontaneously hypertensive rats. Br J Pharmacol. 2013;168:1878–88.
Anwar-mohamed A, Zordoky BN, Aboutabl ME, El-Kadi AO. Alteration of cardiac cytochrome P450-mediated arachidonic acid metabolism in response to lipopolysaccharide-induced acute systemic inflammation. Pharmacol Res. 2010;61:410–8.
Theken KN, Deng Y, Kannon MA, Miller TM, Poloyac SM, Lee CR. Activation of the acute inflammatory response alters cytochrome P450 expression and eicosanoid metabolism. Drug Metab Dispos. 2011;39:22–9.
Bernard NJ, O’Neill LA. Mal, more than a bridge to MyD88. IUBMB Life. 2013;65:777–86.
Gupta SC, Sundaram C, Reuter S, Aggarwal BB. Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta. 2010;1799:775–87.
Landström M. The TAK1-TRAF6 signalling pathway. Int J Biochem Cell Biol. 2010;42:585–9.
Liu SF, Malik AB. NF-kappa B activation as a pathological mechanism of septic shock and inflammation. Am J Physiol. 2006;290:L622–45.
Lu YC, Yeh WC, Ohashi PS. LPS/TLR4 signal transduction pathway. Cytokine. 2008;42:145–51.
Haddad JJ, Abdel-Karim NE. NF-κB cellular and molecular regulatory mechanisms and pathways: therapeutic pattern or pseudoregulation? Cell Immunol. 2011;271:5–14.
Ferreira AM, Minarrieta L, Lamas Bervejillo M, Rubbo H. Nitro-fatty acids as novel electrophilic ligands for peroxisome proliferator-activated receptors. Free Radic Biol Med. 2012;53:1654–63.
Grygiel-Gorniak B. Peroxisome proliferator-activated receptors and their ligands: nutritional and clinical implications-a review. Nutr J. 2014;13(17):23.
Neher MD1, Weckbach S, Huber-Lang MS, Stahel PF. New insights into the role of peroxisome proliferator-activated receptors in regulating the inflammatory response after tissue injury. PPAR Res. 2012;2012:728461.
Wright MB, Bortolini M, Tadayyon M, Bopst M. Minireview: challenges and opportunities in development of PPAR agonists. Mol Endocrinol. 2014;28:1756–68.
von Knethen A, Soller M, Brune B. Peroxisome proliferator-activated receptor gamma (PPAR gamma) and sepsis. Arch Immunol Ther Exp. 2007;55:19–25.
Aggarwal A, Agrawal DK. Importins and exportins regulating allergic immune responses. Mediators Inflamm. 2014;2014:476357.
Cautain B, Hill R, de Pedro N, Link W. Components and regulation of nuclear transport processes. FEBS J. 2015;282:445–62.
Tran EJ, King MC, Corbett AH. Macromolecular transport between the nucleus and the cytoplasm: advances in mechanism and emerging links to disease. Biochim Biophys Acta. 2014;1843:2784–95.
Cuez T, Korkmaz B, Buharalioglu CK, Sahan-Firat S, Falck J, Malik KU, Tunctan B. A synthetic analogue of 20-HETE, 5,14-HEDGE, reverses endotoxin-induced hypotension via increased 20-HETE levels associated with decreased iNOS protein expression and vasodilator prostanoid production in rats. Basic Clin Pharmacol. 2010;106:378–88.
Sari AN, Korkmaz B, Serin MS, Kacan M, Unsal D, Buharalioglu CK, Sahan Firat S, Manthati VL, Falck JR, Malik KU, Tunctan B. Effects of 5,14-HEDGE, a 20-HETE mimetic, on lipopolysaccharide-induced changes in MyD88/TAK1/IKKβ/IκB-α/NF-κB pathway and circulating miR-150, miR-223, and miR-297 levels in a rat model of septic shock. Inflam Res. 2014;63:741–56.
Tunctan B, Korkmaz B, Buharalioglu CK. Sahan Firat S, Anjaiah S, Falck J, Roman RJ, Malik KU. A 20-HETE agonist, N-[20-hydroxyeicosa-5(Z),14(Z)-dienoyl]glycine, opposes the fall in blood pressure and vascular reactivity in endotoxin-treated rats. Shock. 2008;30:329–35.
Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK, Sahan-Firat S, Cuez T, Schunck WH, Manthati VL, Falck JR, Malik KU. Contribution of iNOS/sGC/PKG pathway, COX-2, CYP4A1, and gp91phox to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against vasodilation, hypotension, tachycardia, and inflammation in a rat model of septic shock. Nitric Oxide. 2013;33:18–41.
Tunctan B, Korkmaz B, Sari AN, Kacan M, Unsal D, Serin MS, Buharalioglu CK, Sahan-Firat S, Cuez T, Schunck WH, Falck JR, Malik KU. 5,14-HEDGE, a 20-HETE mimetic, reverses hypotension and improves survival in a rodent model of septic shock: contribution of soluble epoxide hydrolase, CYP2C23, MEK1/ERK1/2/IKKβ/IκB-α/NF-κB pathway, and proinflammatory cytokine formation. Prostaglandins Other Lipid Mediat. 2013;102–103:31–41.
Tunctan B, Korkmaz B, Yildirim H, Tamer L, Atik U, Buharalioglu C. Increased production of nitric oxide contributes to renal oxidative stress in endotoxemic rat. Am J Infect Dis. 2005;1:111–5.
Gadjeva M, Tomczak MF, Zhang M, Wang YY, Dull K, Rogers AB, Erdman SE, Fox JG, Carroll M, Horwitz BH. A role for NF-kappa B subunits p50 and p65 in the inhibition of lipopolysaccharide-induced shock. J Immunol. 2004;173:5786–93.
Ghosh S, Karin M. Missing pieces in the NF-kappaB puzzle. Cell. 2002;109:S81–96.
Birbach A, Gold P, Binder BR, Hofer E, de Martin R, Schmid JA. Signaling molecules of the NF-kappa B pathway shuttle constitutively between cytoplasm and nucleus. J Biol Chem. 2002;277:10842–51.
Huang TT, Kudo N, Yoshida M, Miyamoto S. A nuclear export signal in the N-terminal regulatory domain of IkappaBalpha controls cytoplasmic localization of inactive NF-kappaB/IkappaBalpha complexes. Proc Natl Acad Sci USA. 2000;97:1014–9.
Johnson C, Van Antwerp D, Hope TJ. An N-terminal nuclear export signal is required for the nucleocytoplasmic shuttling of IkappaBalpha. EMBO J. 1999;18:6682–93.
Shirakawa F, Mizel SB. In vitro activation and nuclear translocation of NF-kappa B catalyzed by cyclic AMP-dependent protein kinase and protein kinase C. Mol Cell Biol. 1989;9:2424–30.
Fagerlund R, Melén K, Cao X, Julkunen I. NF-kappaB p52, RelB and c-Rel are transported into the nucleus via a subset of importin alpha molecules. Cell Signal. 2008;20:1442–51.
Fagerlund R, Kinnunen L, Köhler M, Julkunen I, Melen K. NF-{kappa}B is transported into the nucleus by importin {alpha}3 and importin {alpha}4. J Biol Chem. 2005;280:15942–51.
Zerfaoui M, Errami Y, Naura AS, Suzuki Y, Kim H, Ju J, Liu T, Hans CP, Kim JG, Abd Elmageed ZY, Koochekpour S, Catling A, Boulares AH. Poly(ADP-ribose) polymerase-1 is a determining factor in Crm1-mediated nuclear export and retention of p65 NF-kappa B upon TLR4 stimulation. J Immunol. 2010;185:1894–902.
Iida A, Yoshidome H, Shida T, Takano S, Takeuchi D, Kimura F, Shimizu H, Ohtsuka M, Miyazaki M. Hepatocyte nuclear factor-kappa beta (NF-kappaB) activation is protective but is decreased in the cholestatic liver with endotoxemia. Surgery. 2010;148:477–89.
Jiang Q, Liu P, Wu X, Liu W, Shen X, Lan T, Xu S, Peng J, Xie X, Huang H. Berberine attenuates lipopolysaccharide-induced extracellular matrix accumulation and inflammation in rat mesangial cells: involvement of NF-κB signaling pathway. Mol Cell Endocrinol. 2011;331:34–40.
Kwon WY, Suh GJ, Kim KS, Kwak YH. Niacin attenuates lung inflammation and improves survival during sepsis by downregulating the nuclear factor-κB pathway. Crit Care Med. 2011;39:328–34.
Meyer-Schwesinger C, Dehde S, von Ruffer C, Gatzemeier S, Klug P, Wenzel UO, Stahl RA, Thaiss F, Meyer TN. Rho kinase inhibition attenuates LPS-induced renal failure in mice in part by attenuation of NF-kappaB p65 signaling. Am J Physiol. 2009;296:F1088–99.
Zhang X, Song Y, Ci X, An N, Ju Y, Li H, Wang X, Han C, Cui J, Deng X. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57:524–9.
Mandard S, Patsouris D. Nuclear control of the inflammatory response in mammals by peroxisome proliferator-activated receptors. PPAR Res. 2013;2013:613864.
Ishizuka T, Ito O, Tan L, Ogawa S, Kohzuki M, Omata K, Takeuchi K, Ito S. Regulation of cytochrome P-450 4A activity by peroxisome proliferator-activated receptors in the rat kidney. Hypertens Res. 2003;26:929–36.
Ito O, Nakamura Y, Tan L, Ishizuka T, Sasaki Y, Minami N, Kanazawa M, Ito S, Sasano H, Kohzuki M. Expression of cytochrome P-450 4 enzymes in the kidney and liver: regulation by PPAR and species-difference between rat and human. Mol Cell Biochem. 2006;284:141–8.
Lee DL, Wilson JL, Duan R, Hudson T, El-Marakby A. Peroxisome proliferator-activated receptor-α activation decreases mean arterial pressure, plasma interleukin-6, and COX-2 while increasing renal CYP4A expression in an acute model of DOCA-salt hypertension. PPAR Res. 2011;2011:502631.
Ng VY, Huang Y, Reddy LM, Falck JR, Lin ET, Kroetz DL. Cytochrome P450 eicosanoids are activators of peroxisome proliferator-activated receptor alpha. Drug Metab Dispos. 2007;35:1126–34.
Zhou Y, Luo P, Chang HH, Huang H, Yang T, Dong Z, Wang CY, Wang MH. Clofibrate attenuates blood pressure and sodium retention in DOCA-salt hypertension. Kidney Int. 2008;74:1040–8.
Liang CJ, Tseng CP, Yang CM, Ma YH. 20-Hydroxyeicosatetraenoic acid inhibits ATP-induced COX-2 expression via peroxisome proliferator activator receptor-α in vascular smooth muscle cells. Br J Pharmacol. 2011;163:815–25.
Barclay TB, Peters JM, Sewer MB, Ferrari L, Gonzalez FJ, Morgan ET. Modulation of cytochrome P-450 gene expression in endotoxemic mice is tissue specific and peroxisome proliferator-activated receptor-alpha dependent. J Pharmacol Exp Ther. 1999;290:1250–7.
Iwamoto F, Umemoto T, Motojima K, Fujiki Y. Nuclear transport of peroxisome-proliferator activated receptor & alpha. J Biochem. 2011;149:311–9.
Umemoto T, Fujiki Y. Ligand-dependent nucleo-cytoplasmic shuttling of peroxisome proliferator-activated receptors. PPARα and PPARγ. Genes Cells. 2012;17:576–96.
von Knethen A, Tzieply N, Jennewein C, Brüne B. Casein-kinase-II-dependent phosphorylation of PPARgamma provokes CRM1-mediated shuttling of PPARgamma from the nucleus to the cytosol. J Cell Sci. 2010;123:192–201.
Forwood JK, Lam MH, Jans DA. Nuclear import of Creb and AP-1 transcription factors requires importin-beta 1 and Ran but is independent of importin-alpha. Biochemistry. 2001;40:5208–17.
Schreck I, Al-Rawi M, Mingot JM, Scholl C, Diefenbacher ME, O’Donnell P, Bohmann D, Weiss C. c-Jun localizes to the nucleus independent of its phosphorylation by and interaction with JNK and vice versa promotes nuclear accumulation of JNK. Biochem Biophys Res Commun. 2011;407:735–40.
Waldmann I, Wälde S, Kehlenbach RH. Nuclear import of c-Jun is mediated by multiple transport receptors. J Biol Chem. 2007;282:27685–892.
Burgermeister E, Seger R. MAPK kinases as nucleo-cytoplasmic shuttles for PPARgamma. Cell Cycle. 2007;6:1539–48.
Ramer R, Walther U, Borchert P, Laufer S, Linnebacher M, Hinz B. Induction but not inhibition of COX-2 confers human lung cancer cell apoptosis by celecoxib. J Lipid Res. 2013;54:3116–29.
Chen HH, Chen TW, Lin H. Pravastatin attenuates carboplatin-induced nephrotoxicity in rodents via peroxisome proliferator-activated receptor alpha-regulated heme oxygenase-1. Mol Pharmacol. 2010;78:36–45.
Shibuya A, Wada K, Nakajima A, Saeki M, Katayama K, Mayumi T, Kadowaki T, Niwa H, Kamisaki Y. Nitration of PPARgamma inhibits ligand-dependent translocation into the nucleus in a macrophage-like cell line, RAW 264. FEBS Lett. 2002;525:43–7.
Xue H, Chen B, Fan Y, Palikhe M, Li Y. The inhibitory effect of polypeptide cSN50 on alcoholic hepatic injuries through blocking the binding of NF-κB to importin α. Scand J Gastroenterol. 2011;46:931–40.
Acknowledgments
Financial support was provided by grants from Mersin University (BAP-SBE FB [SPS] 2014-3 YL, the Robert A. Welch Foundation (I-0011), and NIH (HL111392). The results of this study were included in the Master’s Thesis of Pharm. M.S. Sefika Pinar Senol.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Senol, S.P., Temiz, M., Guden, D.S. et al. Contribution of PPARα/β/γ, AP-1, importin-α3, and RXRα to the protective effect of 5,14-HEDGE, a 20-HETE mimetic, against hypotension, tachycardia, and inflammation in a rat model of septic shock. Inflamm. Res. 65, 367–387 (2016). https://doi.org/10.1007/s00011-016-0922-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-016-0922-5