Abstract
Background and objective
Asthma is an inflammatory disease of the lungs that is characterised by increased inflammatory cell infiltration into the airways and poor respiratory function. Ivermectin is a semi-synthetic derivative of a family of macrocyclic lactones that shows broad-spectrum anti-parasitic activity. This drug has been shown to possess anti-inflammatory activity, but whether it can be used in asthma treatment has not yet been investigated. In this study, we aimed to investigate the inhibitory effects of ivermectin on allergic asthma symptoms in mice.
Methods and results
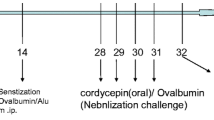
We used a mouse asthma model, in which allergic airway inflammation and airway remodelling were induced by ovalbumin (OVA) sensitisation and challenge. Ivermectin or PBS treatment was administered 1 h before OVA challenge. Ivermectin at 2 mg/kg significantly diminished recruitment of immune cells, production of cytokines in the bronchoalveolar lavage fluids and secretion of OVA-specific IgE and IgG1 in the serum. Histological studies indicated that ivermectin suppressed mucus hypersecretion by goblet cells in the airway.
Conclusions
This is the first study to demonstrate that ivermectin is an effective suppressor of inflammation and may be efficacious in the treatment of non-infectious airway inflammatory diseases such as allergic asthma.






Similar content being viewed by others
Abbreviations
- BALF:
-
Bronchoalveolar lavage fluids
- Cdyn:
-
Lung compliance
- DEX:
-
Dexamethasone
- H&E:
-
Hematoxylin and eosin
- IVER:
-
Ivermectin
- OVA:
-
Ovalbumin
- PAS:
-
Periodic acid-Schiff
- PBS:
-
Phosphate-buffered saline
- RL:
-
Airway resistance
References
Elias JA, Lee CG, Zheng T, Ma B, Homer RJ, Zhu Z. New insights into the pathogenesis of asthma. J Clin Invest. 2003;111:291–7.
Wills-Karp M. Immunologic basis of antigen-induced airway hyperresponsiveness. Annu Rev Immunol. 1999;17:255–81.
Anderson GP, Coyle AJ. Th2 and ‘Th2-like’ cells in allergy and asthma: pharmacological perspectives. Trends Pharmacol Sci. 1994;15:324–32.
Braman SS. The global burden of asthma. Chest. 2006;130:4–12.
Bryskier A, Agouridas C, Chantot JF. New medical targets for macrolides. Exp Opin Invest Drugs. 1994;3:405–10.
Tarayre JP, Aliaga M, Barbara M, Villanova G, Ballester R, Tisne-Versailles J, et al. Cutaneously applied erythromycin base reduces various types of inflammatory reactions in mouse ear. Int J Tiss Reac. 1987;4:77–85.
Mikasa K, Kita E, Sawaki M, Kunimatsu M, Hamada K, Konishi M, et al. The anti-inflammatory effect of erythromycin in zymosan-induced peritonitis of mice. J Antimicrob Chemother. 1992;30:339–48.
Agen C, Danesi R, Blandizzi C, Costa M, Stacchini B, Favini P, et al. Macrolide antibiotics as antiinflammatory agents: roxithromycin in an unexpected role. Agents Actions. 1993;38:85–90.
Giamarellos-Bourboulis EJ. Macrolides beyond the conventional antimicrobials: a class of potent immunomodulators. Int J Antimicrob Agents. 2008;31:12–20.
Hrvačić B, Bošnjak B, Bosnar M, Ferenčić Ž, Glojnarić I, Eraković Haber V. Clarithromycin suppresses airway hyperresponsiveness and inflammation in mouse models of asthma. Eur J Pharmacol. 2009;15:236–43.
Beigelman A, Gunsten S, Mikols CL, Vidavsky I, Cannon CL, Brody SL, et al. Azithromycin attenuates airway inflammation in a noninfectious mouse model of allergic asthma. Chest. 2009;136(2):498–506.
Caumes E, Danis M. New indications of ivermectin. Rev Med Interne. 2001;22:379–84.
Stankiewicz M, Cabaj W, Jonas WE, Moore LG, Millar K, Ng Chie W. Influence of ivermectin on cellular and humoral immune responses of lambs. Vet Immunol Immunopathol. 1995;44:347–58.
Zhang X, Song Y, Xiong H, Ci X, Li H, Yu L, et al. Inhibitory effects of ivermectin on nitric oxide and prostaglandin E2 production in LPS-stimulated RAW 264.7 macrophages. Int J Immunopharmacol. 2009;9:354–9.
Zhang X, Song Y, Ci X, An N, Ju Y, Li H, et al. Ivermectin inhibits LPS-induced production of inflammatory cytokines and improves LPS-induced survival in mice. Inflamm Res. 2008;57:524–9.
Van Rijt LS, Kuipers H, Vos N, Hijdra D, Hoogsteden HC, Lambrecht BN. A rapid flow cytometric method for determining the cellular composition of bronchoalveolar lavage fluid cells in mouse models of asthma. J Immunol Methods. 2004;288:111–21.
Zhou J, Kang Z, Xie Q, Liu C, Lou S, Chen Y, et al. Rapid nongenomic effects of glucocorticoids on allergic asthma reaction in the guinea pig. J Endocrinol. 2003;177:R1–4.
Pene J, Rousset F, Briere F, Chretien I, Bonnefoy JY, Spits H, et al. IgE production by normal human lymphocytes is induced by interleukin 4 and suppressed by interferons a, g and prostaglandin E2. Proc Nat Acad Sci USA. 1988;85:6880–4.
Umetsu DT, DeKruyff RH. TH1 and TH2 CD4+ cells in human allergic diseases. J Allergy Clin Immunol. 1997;100:1–6.
Corry DB, Grunig G, Hadeiba H, Kurup VP, Warnock ML, Sheppard D, et al. Requirementsfor allergen-induced airway hyperreactivity in T and B cell-deficient mice. Mol Med. 1998;4:344–55.
Curtis JL, Byrd PK, Warnock ML, Kaltreider HB. Requirement of CD4-positive T cells for cellular recruitment to the lungs of mice in response to a particulate intratracheal antigen. J Clin Invest. 1991;88:1244–54.
Kips JC. Cytokines in asthma. Eur Respir J. 2001;18:24–33.
Wills-Karp M. Interleukin-13 in asthma pathogenesis. Immunol Rev. 2004;202:175–90.
Ngoc LP, Gold DR, Tzianabos AO, Weiss ST, Celedon JC. Cytokines, allergy, and asthma. Curr Opin Allergy Clin Immunol. 2005;5:161–6.
Wills-Karp M, Luyimbazi J, Xu X, Schofield B. Interleukin-13: central mediator of allergic asthma. Science. 1998;282:2258–61.
Purkerson J, Isakson P. A two-signal model for regulation of immunoglobulin isotype switching. FASEB J. 1992;6:3245–52.
Kimber I, Stone S, Dearman RJ. Assessment of the inherent allergenic potential of proteins in mice. Environ Health Perspect. 2003;111:227–31.
Abu-Ghazaleh RI, Kita H, Gleich GJ. Eosinophil activation and function in health and disease. Immunol Ser. 1992;57:137–67.
Coffman RL, Seymour BW, Hudak S, Jackson J, Rennick D. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science. 1898;245:308–10.
Kopf M, Brombacher F, Hodgkin PD, Ramsay AJ, Milbourne EA, Dai WJ, et al. IL-5-deficient mice have a developmental defect in CD5+ B-1 cells and lack eosinophilia but have normal antibody and cytotoxic T cell responses. Immunity. 1996;4:15–24.
Lee JJ, McGarry MP, Farmer SC, Denzler KL, Larson KA, Carrigan PE, et al. Interleukin-5 expression in the lung epithelium of transgenic mice leads to pulmonary changes pathognomonic of asthma. J Exp Med. 1997;185:2143–56.
Fostera PS, Martinez-Moczygembab M, Hustonb DP, Corry DB. Interleukins-4, -5, and -13: emerging therapeutic targets in allergic disease. Pharmacol Ther. 2002;94:253–64.
Toelle BG, Peat JK, Salome CM. Toward a definition of asthma for epidemiology. Am Rev Respir Dis. 1992;146:633–7.
Peat J, Toelle B, Salome C. Predictive nature of bronchial responsiveness and respiratory symptoms in a one year cohort study of Sydney schoolchildren. Eur Respir J. 1993;6:662–9.
Zhu Z, Homer RJ, Wang Z. Pulmonary expression of interleukin-13 causes inflammation, mucus hypersesretion, subepithelial fibrosis, physiologic abnormalities, and eotaxin production. J Clin Invest. 1999;103:779–88.
Flohé L, Brigelius-Flohé R, Saliou C, Traber MG, Packer L. Redox regulation of NF-kappa B activation. Free Radic Biol Med. 1997;22:1115–26.
Vanden Berghe W, Plaisance S, Boone E, De Bosscher K, Schmitz ML, Fiers W. p38 and extracellular signal-regulated kinase mitogen-activated protein kinase pathways are required for nuclear factor-kappaB p65 transactivation mediated by tumor necrosis factor. J Biol Chem. 1998;273:3285–90.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Parnham.
Rights and permissions
About this article
Cite this article
Yan, S., Ci, X., Chen, N. et al. Anti-inflammatory effects of ivermectin in mouse model of allergic asthma. Inflamm. Res. 60, 589–596 (2011). https://doi.org/10.1007/s00011-011-0307-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-011-0307-8