Abstract
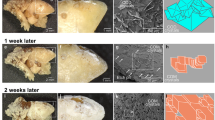
Injection of CaATP solutions subcutaneously in rats resulted in the formation of white, circumscribed, gritty plaques containing amorphous calcium phosphate (Ca/P 1.3–1.5). Dense, rod-shaped deposits were oriented along the fibrous elements and surrounding blood vessels in the subcutaneous connective tissue. The response was dose related, the amount of plaque calcium being about 100 times the amount injected. These results suggest that the calcium phosphate formed from CaATP in the ATPase active subcutaneous tissue may nucleate crystallization. Injection of a solution of CaCl2 having the same concentration as the calcium in the CaATP solution, or of a sodium phosphate solution having the same concentration of phosphorus as the CaATP, did not produce plaques. However, injection of a solution containing both Ca2+ and PO 3−4 produced plaques, although smaller than those formed by injection of CaATP.
Using as standard CaATP injection dose containing increasing amounts of magnesium, plaques were formed whose size and ashed weight were inversely proportional to the Mg/Ca ratio in the solution injected. In this system, the results suggest that the local ratio of Mg/Ca may affect the calcification process. A corollary to this conclusion is that it may be possible to inhibit soft tissue calcification through control of the local Mg/Ca ratios.
Résumé
L'injection sous-cutanée de solutions de Ca ATP provoque, chez le Rat, la formation de plaques rugueses et circonscrites, de couleur blanche, contenant du phosphate de calcium amorphe (Ca/P 1.3, 1.5). Des dépôts denses, en forme de bâtonnets, orientés le long des éléments fibreux, entourent les vaisseaux sanguins et sont situés dans le tissu conjonctif souscutané. La réaction observée est proportionnelle à la dose utilisée, la quantité de calcium de la plaque étant environ 100 fois celle de la quantité injectée. Ces résultats semblent indiquer que le phosphate de calcium, formé à partir du Ca ATP, dans le tissu sous-cutané actif en ATP-ase, est susceptible de provoquer un phénomène de cristallisation par nucléation. L'injection d'une solution de Ca Cl2, ayant la même concentration en calcium que la solution Ca ATP ou d'une solution de phosphate de sodium, ayant la même concentration en phosphore que Ca ATP, ne provoque pas de plaques. Cependant, l'injection d'une solution contenant du Ca2+ et du PO 3−4 forme de la plaque, en quantité inférieure à celle provoquée par l'injection de Ca ATP.
A l'aide d'une dose d'injection type, contenant des concentrations accrues de magnesium, on observe la formation de plaques dont la taille et le poids en cendres sont inversement proportionnels au rapport Mg/Ca de la solution injectée. Dans ce système, il semble que le rapport Mg/Ca puisse affecter le processus de calcification et qu'il puisse aussi inhiber la calcification de tissus mous, en controlant les rapports locaux Mg/Ca.
Zusammenfassung
Die subkutane Injection von CaATP-Lösungen in Ratten führte zur Bildung von weißen, umschriebenen, sandigen Plättchen, welche amorphes Calciumphosphat enthielten (Ca/P 1,3; 1,5). Dichte, rutenförmige Ablagerungen fanden sich entlang den fibrösen Elementen und umgaben Blutgefäße im subkutanen Bindegewebe. Diese Reaktion hing von der Dosis ab; die Menge des Calciums in den Plättchen entsprach etwa der hundertfachen eingespritzten Menge. Diese Ergebnisse lassen vermuten, daß das Calciumphosphat, welches im ATPase-aktiven subcutanen Gewebe aus CaATP gebildet wurde, als Keim für die Kristallisation wirken kann. Die Injektion einer Lösung von CaCl2, welche dieselbe Konzentration hatte wie das Calcium in der CaATP-Lösung, oder einer Natriumphosphatlösung, welche dieselbe Phosphorkonzentration wie CaATP hatte, erzeugte keine Plättchen. Hingegen erzeugte die Injektion einer Lösung, welche Ca2+ und PO 3−4 enthielt, Plättchen, die jedoch kleiner waren als diejenigen nach der Injektion von CaATP.
Bei Verwendung einer Standard-Injektionsdosis, welche zunehmende Mengen von Magnesium enthielt, wurden Plättchen gebildet, deren Größe und Aschgewicht umgekehrt proportional zum Mg/Ca-Verhältnis in der eingespritzten Lösung waren. Die Ergebnisse bei diesem System lassen vermuten, daß das lokale Mg/Ca-Verhältnis den Kalzifikationsprozeß beein-flussen kann. Aus diesen Befunden folgt, daß es möglich sein könnte, die Weichteilverkalkung durch die Kontrolle der lokalen Mg/Ca-Verhältnisse zu hemmen.
Similar content being viewed by others
References
Bachra, B. N., Fischer, H. R. A.: The effect of some inhibitors on the nucleation and crystal growth of apatite. Calc. Tiss. Res.3, 348–357 (1969).
Brown, W. E.: Private communication (1971).
Fernley, H. N., Walker, P. G.: Studies on alkaline phosphatase. Inhibition by phosphate derivatives and the substrate specificity. Biochem. J.104, 1011–1018 (1967).
Fiske, C. H., Subbarow, Y.: The colorimetric determination of phosphorus. J. biol. Chem.66, 375–400 (1925).
Gershoff, S. N., Prien, E. L.: Effect of daily MgO and vitamin B6 administration to patients with recurring calcium oxalate kidney stones. Amer. J. clin. Nutr.20, 393–399 (1967).
Gotz, F., Wolfer, E., Vary, L.: Drinking water and renal calculus formation. Acta med. Acad. Sci. hung.24, 123–127 (1967).
Hegyeli, A. F., Johnsson-Hegyeli, R.: Reflected light interference microscopy of living and fixed biological suriaces. Nature213, 417–418 (1967).
Leonard, F., Scullin, R. I.: New mechanism for calcification of skeletal tissue. Nature224, 1113–1115 (1969).
Leonard, F., Wade, C. W. R., Hegyeli, A. F.: Mechanism of calcification. Clin. Orthop.78, 168–172 (1971).
Moore, C. A., Bunce, G. E.: Reduction in frequency of renal calculus formation by oral magnesium administration. Invest. Urol.2, 7–13 (1964).
Morris, M. L., Jr., Featherston, W. R., Phillips, P. H., McNutt, S. H.: Influence of lactose and dried skim milk upon the magnesium deficiency syndrome in the dog. II. Pathological changes. J. Nutr.79, 437–441 (1963).
Nanninga, L. B.: Formation constants and hydrolysis at 100° of calcium and magnesium complexes of adenosinetri-, di- and monophosphate. J. Phys. Chem.61, 1144–1149 (1957).
Neuman, W. F., Mulryan, B. J.: Synthetic hydroxypatite crystals. IV. Magnesium incorporation. Calc. Tiss. Res.7, 133–138 (1971).
Pak, C. Y. C., Diller, E. C.: Ionic interaction with bone mineral. V. Effect of Mg2+, Citrate3− F− and SO 2−4 on the solubility, dissolution and growth of bone mineral. Calc. Tiss. Res.4, 69–77 (1969).
Sobel, A. E.: Local factors in the mechanism of calcification. Ann. N.Y. Acad. Sci.60, 713–732 (1955).
Termine, J. D., Posner, A. S.: Calcium phosphate formationin vitro. I. Factors affecting initial phase separation. Arch. Biochem.140, 307–317 (1970).
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Leonard, F., Boke, J.W., Ruderman, R.J. et al. Initiation and inhibition of subcutaneous calcification. Calc. Tis Res. 10, 269–279 (1972). https://doi.org/10.1007/BF02012558
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02012558