Summary
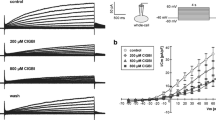
Nitrogen mustard (N-mustard) inhibits the ouabain-sensitive and the furosemide-sensitive Rb uptake of Ehrlich ascites tumor cells, whereas the transport, which is resistant to both inhibitors, is not affected by the alkylating agent. At N-mustard concentrations below 10 μM, the reduction in Rb uptake is predominantly due to an interference with the furosemide-sensitive system. The dose response curve for the inhibition by N-mustard of the furosemide-sensitive Rb uptake closely parallels the dose response curve for the anti-tumor activity of the alkylating drug. This is in contrast to the behaviour of the ouabain-sensitive Rb transport.
The inhibition of the furosemide-sensitive Rb uptake is expressed much less in cells which are resistant to N-mustard. The recovery of the furosemide-sensitive transport system after a single exposure to N-mustard is relatively slow and characterized by an initial 4 h lag period, whereas the repair of DNA-interstrand cross-links starts immediately after removal of the drug. At mM concentrations furosemide blocks the multiplication of Ehrlich ascites tumor cells. However, lower concentrations of furosemide which cause a 50% reduction in the furosemide-sensitive Rb uptake do not interfere with cell proliferation. This is in contrast to the behaviour of N-mustard which exerts a clear-cut depression of cell growth at concentrations leading to a 50% inhibition of the furosemide-sensitive Rb transport. It is concluded, therefore, that the inhibition of the furosemide-sensitive system alone is not sufficient to explain the anti-tumor activity of the alkylating agent.
The effect is discussed as part of a more extended N-mustard-induced membrane alteration which may be important for the growth inhibitory effect of the alkylating agent.
Similar content being viewed by others
References
Aggarwal SK, Niroomand-Rad I (1983) Effect of cisplatin on the plasma membrane phosphatase activities in ascites sarcoma 180 cells. A cytochemical study. J Histochem Cytochem 31:307–317
Aiton JF, Chipperfield AR, Lamb JF, Ogden P, Simmons NL (1981) Occurrence of passive furosemide-sensitive transmembrane potassium transport in cultured cells. Biochim Biophys Acta 646:389–398
Baxter MA, Chahwala SB, Hickman JA, Spurgin GE (1982) The effect of nitrogen mustard (HN2) on activities of the plasma membrane of PC6A mouse plasma cytoma cells. Biochem Pharmacol 31:1773–1778
Chipperfield AR (1980) An effect of chloride on (Na+K) co-transport in human red blood cells. Nature 286:281–282
Chipperfield AR (1981) Chloride dependence of furosemide- and phloretin-sensitive passive sodium and potassium fluxes in human red cells. J Physiol 312:435–444
Crane FL, Sun JL, Chou JY, Löw H (1983) Selective adriamycin inhibition of transmembrane redox activity in transformed cells. J Cell Biol 97:71a
Duhm J, Göbel BO (1984) Role of the furosemide-sensitive Na+/K+ transport system in determining the steady-state Na+ and K+ content and volume of human erythrocytes in vitro and in vivo. J Membr Biol 77:243–254
Dunn MJ (1973) Ouabain-uninhibited sodium transport in human erythrocytes: Evidence against a second pump. J Clin Invest 52:658–670
Geck P, Pietrzyk C, Burckhardt BC, Pfeiffer B, Heinz E (1980) Electrically silent cotransport of Na+, K+ and Cl− in Ehrlich cells. Biochim Biophys Acta 600:432–447
Grunicke H, Grünewald K, Helliger W, Scheidl F, Wolff-Schreiner E, Puschendorf B (1983) Inhibition of tumor growth by an alkylation of the plasma membrane. Adv Enzyme Regul 92:21–30
Grunicke H, Hirsch F, Wolf H, Bauer U, Kiefer G (1975) Selective inhibition of thymidine transport at low doses of the alkylating agent trisethyleneiminobenzoquinone (Trenimon). Exp Cell Res 90:357–364
Hoffmann, EK, Sjoholm C, Simonson LO (1983) Na+, Cl− cotransport in Ehrlich ascites tumor cells activated during volume regulation (regulatory volume increase). J Membr Biol 76:269–280
Hoffmann JF, Kregenow FM (1966) The characterization of new energy dependent cation transport processes in red blood cells. Ann NY Acad Sci 137:566–576
Ihlenfeldt M, Gantner G, Harrer M, Puschendorf B, Putzer H, Grunicke H (1981) Interaction of the alkylating antitumor agent 2,3,5-tris(ethyleneimino)-benzoquinone with the plasma membrane of Ehrlich ascites tumor cells. Cancer Res 41:289–293
Kennedy BG, Lever JE (1984) Regulation of Na+, K+-ATPase activity in MDCK kidney epithelial cell cultures: Role of growth state, cyclic AMP, and chemical inducers of dome formation and differentiation. J Cell Physiol 121:51–63
Laris PC, Henius GV (1982) Influence of glucose on Ehrlich cell volume, ion transport, and membrane potential. Am J Physiol 242:C326–332
Lubin F (1982) The potassium sensitivity shift and other matters. In: Boynton AL, McKeehan WL, Whitfield JF (eds) Ions, cell proliferation and cancer. Academic Press, New York, pp 131–150
Nielsen K, Granzow C (1983) Chromosome band pattern of near tetraploid Ehrlich-Lettré mouse ascites cells (ELT Bonn) in vivo and in vitro. Hereditas 98:95–103
Pain VM, Lewis JA, Huvos P, Henshaw EC, Clemens MJ (1980) The effects of amino acid starvation on regulation of polypeptide chain initiation in Ehrlich ascites tumor cells. J Biol Chem 255:1486–1491
Panet R, Fromer J, Atlan H (1982) Differentiation between serum stimulation of ouabain-resistant and sensitive Rb influx in quiescent NIH3T3 cells. J Membr Biol 70:165–169
Rogers KF, Carr BE, Tökes ZA (1983) Cell surface-mediated cytotoxicity of polymer-bound adriamycin against drug-resistant hepatocytes. Cancer Res 43:2741–2748
Schaeffer A, Munter KH, Geck P, Koch GA (1984) Reduction in the activity of the Na+, K+-pump in dimethyl-sulfoxidetreated Friend erythroleukemia cells is not due to partial inactivation of the Na+, K+-ATPase. J Cell Physiol 119:335–350
Sun IL, Crane FL, Chou JY, Öw H, Grebing (1983) Transformed liver cells have modified transplasma membrane redox activity which is sensitive to adriamycin. Biochem Biophys Res Commun 116:210–216
Tritton TR, Hickman JA (1984) Cell surface membranes as a chemotherapeutic target. In: McGuire WL (ed) Chemotherapy, vol 2, Martinus Nijoff, Den Haag (in press)
Tritton TR, Yee G (1982) The anticancer drug adriamycin can be actively cytotoxic without entering cells. Science 217:248–250
Tupper JT (1975) Cation flux in the Ehrlich ascites tumor cell. Evidence for a Na+-for-Na+ and K+-for-K+ exchange diffusion. Biochim Biophys Acta 394:586–596
Ueberschär S, Bakker-Grunwald T (1983) Bumetanide-sensitive potassium transport and volume regulation in turkey erythrocytes. Biochim Biophys Acta 731:243–250
Van den Berg EK, Brazy PC, Huang AT, Dennis VW (1981) Cisplatin-induced changes in sodium, chloride and urea transport by the frog skin. Kidney Int 19:8–14
Wiley JS, Cooper RA (1974) A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest 53:745–755
Wilman DEV, Connors TA (1983) Molecular aspects of anti-cancer drug action. In: Neidle S, Waring MJ (eds) Molecular aspects of anti-cancer drug action. Verlag Chemie GmbH, Weinheim Deerfield Beach Florida Basel, pp 233–282
Wondergem R (1982) Intracellular potassium activity during liver regeneration. In: Boynton AL, McKeehan WL, Whitfield JF (eds) Ions, cell proliferation and cancer. Academic Press, New York, pp 175–186
Zwelling LA, Michaels S, Schwartz H, Dobson PP, Kohn KW (1981) DNA cross-linking as an indicator of sensitivity and resistance of mouse L1210 leukemia to cis-diamminedichloroplatinum (II) and L-phenylalanine mustard. Cancer Res 41:640–649
Author information
Authors and Affiliations
Additional information
These studies were supported by the Austrian Fund for Support of Scientific Research (Fonds zur Förderung der wissenschaftlichen Forschung) Project no. P4690
Rights and permissions
About this article
Cite this article
Doppler, W., Hofmann, J., Oberhuber, H. et al. Nitrogen mustard interference with potassium transport systems in Ehrlich ascites tumor cells. J Cancer Res Clin Oncol 110, 35–41 (1985). https://doi.org/10.1007/BF00402499
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00402499