Abstract
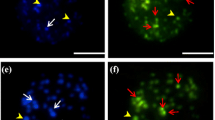
Mitotic chromosomes, interphase cell nuclei, and male meiosis of 41 species representing all vertebrate classes were analyzed with distamycin A/mithramycin counterstaining. The purpose of the study was to recognize differences and common characteristics in the reverse (R) fluorescent banding patterns in the chromosomes of vertebrate species at various stages of evolution. In contrast to the warm-blooded mammals and birds, the euchromatic segments in the chromosomes of most reptiles, amphibians, and fishes contain no multiple fluorescent R-bands. This is thought to be due to the absence of the long homogeneous regions (isochores) in the DNA of the cold-blooded vertebrates. Distamycin A/mithramycin banding specifically reveals the GC-rich constitutive heterochromatin in all vertebrates. In most of the vertebrate chromosomes examined, the heterochromatic regions have opposite staining properties with mithramycin and quinacrine. Mithramycin labels the nucleolus organizer regions very brightly in the karyotypes of fishes, amphibians, reptiles and birds, but not of mammals. The lack of mithramycin fluorescence at the nucleolus organizer regions of mammals is attributed to the relatively low level of redundancy of the GC-rich ribosomal DNA in their genomes. Studies on the various meiotic stages of the cold-blooded vertebrates show that the mithramycin labeling of the nucleolus organizers is independent of their state of activity. This can be confirmed by mithramycin fluorescence at the nucleoli of actinomycintreated cells.
Similar content being viewed by others
References
Appels R (1982) The molecular cytology of wheat-rye hybrids. Int Rev Cytol 80:93–132
Attardi G, Amaldi F (1970) Structure and synthesis of ribosomal RNA. Annu Rev Biochem 39:183–226
Behr W, Honikel K, Hartmann G (1969) Interaction of the RNA polymerase inhibitor chromomycin with DNA. Eur J Biochem 9:82–92
Bickham JW, Baker RJ (1976) Chromosome homology and evolution of emydid turtles. Chromosoma 54:201–219
Bross K, Krone W (1973) Ribosomal cistrons and acrocentric chromosomes in man. Hum Genet 18:71–75
Brown DD, Wensink PC, Jordan E (1972) A comparison of the ribosomal DNA's of Xenopus laevis and Xenopus mulleri: the evolution of tandem genes. J Mol Biol 63:57–73
Caspersson T, Zech L, Johannson C, Modest EJ (1970) Identification of human chromosomes by DNA-binding fluorescent agents. Chromosoma 30:215–227
Chromosome Atlas: fish, amphibians, reptiles and birds (1971) Benirschke K, Hsu TC (eds) Springer, New York Heidelberg Berlin, folio nos. Av-6 and Av-12
Comings DE (1978) Mechanisms of chromosome banding and implications for chromosome structure. Annu Rev Genet 12:25–46
Comings DE (1980) Arrangement of chromatin in the interphase nucleus. Hum Genet 53:131–143
Comings DE, Drets ME (1976) Mechanisms of chromosome banding. IX. Are variations in DNA base composition adequate to account for quinacrine, Hoechst 33258, and daunomycin banding? Chromosoma 52:229–243
Cuny G, Soriano P, Macaya G, Bernardi G (1981) The major components of the mouse and human genomes: preparation, basic properties, and compositional heterogeneity. Eur J Biochem 111:227–233
Deumling B, Greilhuber J (1982) Characterization of heterochromatin in different species of the Scilla siberia group (Liliaceae) by in situ hybridisation of satellite DNA and fluorochrome banding. Chromosoma 84:535–555
Filipski J, Thiery JP, Bernardi G (1973) An analysis of the bovine genome by Cs2SO4/Ag+ density gradient centrifugation. J Mol Biol 80:177–197
Gale EF, Cundliffe E, Reynolds PE, Richmond MH, Waring MJ (1972) The molecular basis of antibiotic action. Wiley, London
Goldberg IH, Friedman PA (1971) Antibiotics and nucleic acids. Annu Rev Biochem 40:775–810
Goodpasture C, Bloom SE (1975) Visualization of nucleolar organizer regions in mammalian chromosomes using silver staining. Chromosoma 53:37–50
Hofgärtner FJ, Schmid M, Krone W, Zenzes MT, Engel W (1979) Pattern of activity of nucleolus organizers during spermatogenesis in mammals as analyzed by silver-staining. Chromosoma 71:197–216
Holmquist G (1988) DNA sequences in G-bands and R-bands. In: Adolph KW (ed) Chromosome and chromatin structure. CRC Press, Boca Raton, in press
Hudson AP, Cuny G, Cortadas J, Haschemeyer AEV, Bernardi G (1980) An analysis of fish genomes by density gradient centrifugation. Eur J Biochem 112:203–210
John B, King M, Schweizer D, Mendelak M (1985) Equilocality of heterochromatin distribution and heterochromatin heterogeneity in acridid grasshoppers. Chromosoma 91:185–200
Jorgenson KF, van de Sande JH, Lin CC (1978) The use of base pair specific DNA binding agents as affinity labels for the study of mammalian chromosomes. Chromosoma 68:287–302
King M, Rofe R (1976) Karyotypic variation in the Australian gekko Phyllodactylus marmoratus (Gray) (Gekkonidae: Reptilia). Chromosoma 54:75–87
Kuro-o M, Ikebe C, Kohno S (1986) Cytogenetic studies of Hynobiidae (Urodela). IV. DNA replication bands (R-banding) in the genus Hynobius and the banding karyotype of Hynobius nigrescens Stejneger. Cytogenet Cell Genet 43:14–18
Latt SA (1977) Fluorescent probes of chromosome structure and replication. Can J Genet Cytol 19:603–623
Latt SA, Munroe SH, Disteche C, Rogers WE, Cassel DM (1977) Uses of fluorescent dyes to study chromosome structure and replication. In: De la Chapelle A, Sorsa M (eds) Chromosomes Today 6. Oxford Chromosome Conference, Edinburgh, pp 27–36
Loidl J (1983) Some features of heterochromatin in wild Allium species. Plant Syst Evol 143:117–131
Macaya G, Thiery JP, Bernardi G (1976) An approach to the organization of eukaryotic genomes at a macromolecular level. J Mol Biol 108:237–254
Mayr B, Schweizer D, Mendelak M, Krutzler J, Schleger W, Kalat M, Auer H (1985) Levels of conservation and variation of heterochromatin and nucleolus organizers in the Bovidae. Can J Genet Cytol 27:665–682
Mayr B, Rab P, Kalat M (1986) Nucleolar organizing regions and counterstain-enhanced fluorescence studies in Cyprinidae of different ploidy level. Genetica 69:111–118
Mayr B, Kalat M, Rab P, Lambron M (1987) Banded karyotypes and specific types of heterochromatin in several species of European percid fishes (Percidea, Pisces). Genetica 75:199–205
Medrano L, Bernardi G, Couturier J, Dutrillaux B, Bernardi G (1988) Chromosome banding and genome compartmentalization in fishes. Chromosoma 96:178–183
Mengden GA, Stock AD (1980) Chromosomal evolution in Serpentes: a comparison of G and C chromosome banding patterns of some colubrid and boid genera. Chromosoma 79:53–64
Miller DA, Dev VG, Tantravahi R, Miller OJ (1976a) Suppression of human nucleolus organizer activity in mouse-human somatic hybrid cells. Exp Cell Res 101:235–243
Miller OJ, Miller DA, Dev VG, Tantravahi R, Croce CM (1976b) Expression of human and suppression of mouse nucleolus organizer activity in mouse-human somatic cell hybrids. Proc Natl Acad Sci USA 73:4531–4535
Moritz C (1984) The evolution of a highly variable sex chromosome in Gehyra purpurascens (Gekkonidae). Chromosoma 90:111–119
Ohno S (1974) Protochordata, cyclostomata and pisces. In: John B (ed) Animal cytogenetics, vol 4: Chordata 1. Gebrüder Borntraeger, Berlin Stuttgart, pp 28–32
Ohno S, Stenius C, Christian LC, Beçak W, Beçak ML (1964) Chromosomal uniformity in the avian subclass Carinatae. Chromosoma 15:280–288
Olofsson B, Bernardi G (1983) Organization of nucleotide sequences in the chicken genome. Eur J Biochem 130:241–245
Owen JJW (1965) Karyotype studies on Gallus domesticus. Chromosoma 16:601–608
Park E-H, Grimm H (1981) Distribution of C-band heterochromatin in the ZW sex chromosomes of European and American eels (Anguillidae, Teleostomi). Cytogenet Cell Genet 31:167–174
Pizon V, Cuny G, Bernardi G (1984) Nucleotide sequence organization in the very small genome of a tetraodontid fish, Arothron diadematus. Eur J Biochem 140:25–30
Sahar E, Latt SA (1978) Enhancement of banding patterns in human metaphase chromosomes by energy transfer. Proc Natl Acad Sci USA 75:5650–5654
Sahar E, Latt SA (1980) Energy transfer and binding competition between dyes used to enhance staining differentiation in metaphase chromosomes. Chromosoma 79:1–28
Schempp W, Schmid M (1981) Chromosome banding in Amphibia. VI. BrdU-replication patterns in Anura and demonstration of XX/XY sex chromosomes in Rana esculenta. Chromosoma 83:697–710
Schmid M (1978) Chromosome banding in Amphibia. I. Constitutive heterochromatin and nucleolus organizer regions in Bufo and Hyla. Chromosoma 66:361–388
Schmid M (1980) Chromosome banding in Amphibia. IV. Differentiation of GCand AT-rich chromosome regions in Anura. Chromosoma 77:83–103
Schmid M, Löser C, Schmidtke J, Engel W (1982) Evolutionary conservation of a common patterns of activity of nucleolus organizers during spermatogenesis in vertebrates. Chromosoma 86:149–179
Schmid M, Vitelli L, Batistoni R (1987) Chromosome banding in Amphibia. XI. Constitutive heterochromatin, nucleolus organizers, 18 S+28 S and 5 S ribosomal RNA genes in Ascaphidae, Pipidae, Discoglossidae and Pelobatidae. Chromosoma 95:271–284
Schnedl W, Abraham R, Förster M, Schweizer D (1981) Differential fluorescent staining of porcine heterochromatin by chromomycin A3/distamycin A/DAPI and D287/170. Cytogenet Cell Genet 31:249–253
Schwarzacher HG, Wolf U (1974) Methods in human cytogenetics. Springer, Berlin Heidelberg New York
Schwarzacher T, Schweizer D (1982) Karyotype analyses and heterochromatin differentiation with Giemsa C-banding and fluorescent counterstaining in Cephalanthera (Orchidaceae). Plant Syst Evol 141:91–113
Schwarzacher T, Mayr B, Schweizer D (1984) Heterochromatin and nucleolus-organizer-region behaviour at male pachytene of Sus scrofa domestica. Chromosoma 91:12–19
Schweizer D (1976) Reverse fluorescent chromosome banding with chromomycin and DAPI. Chromosoma 58:307–324
Schweizer D (1979) Fluorescent chromosome banding in plants: applications, mechanisms, and implications for chromosome structure. In: Davies DR, Hopwood RA (eds) Proc 4th John Innes Symp: The plant genome. John Innes Charity, Norwich, pp 61–72
Schweizer D (1980) Simultaneous fluorescent staining of R-bands and specific heterochromatic regions (DA-DAPI bands) in human chromosomes. Cytogenet Cell Genet 27:190–193
Schweizer D (1981) Counterstain-enhanced chromosome banding. Hum Genet 57:1–14
Schweizer D, Mendelak M, White MJD, Contreras N (1983) Cytogenetics of the parthenogenetic grasshopper Warramaba virgo and its bisexual relatives. X. Patterns of fluorescent banding. Chromosoma 88:227–236
Schweizer D, Loidl J, Hamilton B (1987) Heterochromatin and the phenomenon of chromosome banding. In: Hennig W (ed) Results and problems in cell differentiation 14. Structure and function of eukaryotic chromosomes. Springer, Berlin Heidelberg New York, pp 235–254
Sekiya K, Nakagawa H (1983) Cytogenetics of Xenopus laevis. I. G-banding pattern of Xenopus laevis chromosomes. Experientia 39:786–787
Sinclair JH, Brown DD (1971) Retention of common nucleotide sequences in the ribosomal deoxyribonucleic acid of eukaryotes and some of their physical characteristics. Biochemistry 10:2761–2769
Solleder E, Schmid M (1984) XX/XY-sex chromosomes in Gekko gecko (Sauria, Reptilia). Amphibia-Reptilia 5:339–345
Stock AD, Mengden GA (1975) Chromosome banding pattern conservatism in birds and nonhomology of chromosome banding patterns between birds, turtles, snakes and amphibians. Chromosoma 50:69–77
Sumner AT (1972) A simple technique for demonstrating centromeric heterochromatin. Exp Cell Res 75:304–306
Thiébaud CH (1979a) The intra-nucleolar localization of amplified rDNA in Xenopus laevis oocytes. Chromosoma 73:29–36
Thiébaud CH (1979b) Quantitative determination of amplified rDNA and its distribution during oogenesis in Xenopus laevis. Chromosoma 73:37–44
Thiery JP, Macaya G, Bernardi G (1976) An analysis of eukaryotic genomes by density gradient centrifugation. J Mol Biol 108:219–235
Van de Sande JH, Lin CC, Jorgenson KF (1977) Reverse banding on chromosomes produced by a guanosine-cytosine specific DNA binding antibiotic: olivomycin. Science 195:400–402
Ward DC, Reich E, Goldberg IH (1965) Base specificity in the interaction of polynucleotides with antibiotic drugs. Science 149:1259–1263
Wiberg UH (1983) Sex determination in the European eel (Anguilla anguilla L.). Cytogenet Cell Genet 36:589–598
Zimmer C (1975) Effects of the antibiotics netropsin and distamycin A on the structure and function of nucleic acids. Prog Nucleic Acid Res Mol Biol 15:285–318
Author information
Authors and Affiliations
Additional information
Dedicated to the memory of Professor Dr. Hans Bauer
Rights and permissions
About this article
Cite this article
Schmid, M., Guttenbach, M. Evolutionary diversity of reverse (R) fluorescent chromosome bands in vertebrates. Chromosoma 97, 101–114 (1988). https://doi.org/10.1007/BF00327367
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF00327367