Abstract
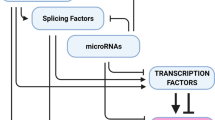
The evolutionary emergence of the mesenchymal phenotype greatly increased the complexity of tissue architecture and composition in early Metazoan species. At the molecular level, an epithelial-to-mesenchymal transition (EMT) was permitted by the innovation of specific transcription factors whose expression is sufficient to repress the epithelial transcriptional program. The reverse process, mesenchymal-to-epithelial transition (MET), involves direct inhibition of EMT transcription factors by numerous mechanisms including tissue-specific MET-inducing transcription factors (MET-TFs), micro-RNAs, and changes to cell and tissue architecture, thus providing an elegant solution to the need for tight temporal and spatial control over EMT and MET events during development and adult tissue homeostasis.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Rodriguez-Boulan E, Macara IG (2014) Organization and execution of the epithelial polarity programme. Nat Rev Mol Cell Biol 15(4):225–242. https://doi.org/10.1038/nrm3775
Campbell K, Casanova J (2016) A common framework for EMT and collective cell migration. Development 143(23):4291–4300. https://doi.org/10.1242/dev.139071
Huang RY, Guilford P, Thiery JP (2012) Early events in cell adhesion and polarity during epithelial-mesenchymal transition. J Cell Sci 125(Pt 19):4417–4422. https://doi.org/10.1242/jcs.099697
Pei D, Shu X, Gassama-Diagne A, Thiery JP (2019) Mesenchymal-epithelial transition in development and reprogramming. Nat Cell Biol 21(1):44–53. https://doi.org/10.1038/s41556-018-0195-z
Takai Y, Nakanishi H (2003) Nectin and afadin: novel organizers of intercellular junctions. J Cell Sci 116(Pt 1):17–27
Rikitake Y, Mandai K, Takai Y (2012) The role of nectins in different types of cell-cell adhesion. J Cell Sci 125(Pt 16):3713–3722. https://doi.org/10.1242/jcs.099572
Roignot J, Peng X, Mostov K (2013) Polarity in mammalian epithelial morphogenesis. Cold Spring Harb Perspect Biol 5(2). https://doi.org/10.1101/cshperspect.a013789
Nakaya Y, Sukowati EW, Wu Y, Sheng G (2008) RhoA and microtubule dynamics control cell-basement membrane interaction in EMT during gastrulation. Nat Cell Biol 10(7):765–775. https://doi.org/10.1038/ncb1739
Eckert JJ, Fleming TP (2008) Tight junction biogenesis during early development. Biochim Biophys Acta 1778(3):717–728. https://doi.org/10.1016/j.bbamem.2007.09.031
Frisch SM (1997) The epithelial cell default-phenotype hypothesis and its implications for cancer. Bioessays 19(8):705–709. https://doi.org/10.1002/bies.950190811
Acloque H, Adams MS, Fishwick K, Bronner-Fraser M, Nieto MA (2009) Epithelial-mesenchymal transitions: the importance of changing cell state in development and disease. J Clin Invest 119(6):1438–1449. https://doi.org/10.1172/JCI38019
McMahon AP (2016) Development of the mammalian kidney. Curr Top Dev Biol 117:31–64. https://doi.org/10.1016/bs.ctdb.2015.10.010
Inman JL, Robertson C, Mott JD, Bissell MJ (2015) Mammary gland development: cell fate specification, stem cells and the microenvironment. Development 142(6):1028–1042. https://doi.org/10.1242/dev.087643
Ewald AJ, Huebner RJ, Palsdottir H, Lee JK, Perez MJ, Jorgens DM, Tauscher AN, Cheung KJ, Werb Z, Auer M (2012) Mammary collective cell migration involves transient loss of epithelial features and individual cell migration within the epithelium. J Cell Sci 125(Pt 11):2638–2654. https://doi.org/10.1242/jcs.096875
Perez-Pomares JM, Munoz-Chapuli R (2002) Epithelial-mesenchymal transitions: a mesodermal cell strategy for evolutive innovation in metazoans. Anat Rec 268(3):343–351. https://doi.org/10.1002/ar.10165
Carninci P, Sandelin A, Lenhard B, Katayama S, Shimokawa K, Ponjavic J, Semple CA, Taylor MS, Engstrom PG, Frith MC, Forrest AR, Alkema WB, Tan SL, Plessy C, Kodzius R, Ravasi T, Kasukawa T, Fukuda S, Kanamori-Katayama M, Kitazume Y, Kawaji H, Kai C, Nakamura M, Konno H, Nakano K, Mottagui-Tabar S, Arner P, Chesi A, Gustincich S, Persichetti F, Suzuki H, Grimmond SM, Wells CA, Orlando V, Wahlestedt C, Liu ET, Harbers M, Kawai J, Bajic VB, Hume DA, Hayashizaki Y (2006) Genome-wide analysis of mammalian promoter architecture and evolution. Nat Genet 38(6):626–635. https://doi.org/10.1038/ng1789
Behrens J, Lowrick O, Klein-Hitpass L, Birchmeier W (1991) The E-cadherin promoter: functional analysis of a G.C-rich region and an epithelial cell-specific palindromic regulatory element. Proc Natl Acad Sci U S A 88(24):11495–11499. https://doi.org/10.1073/pnas.88.24.11495
Ringwald M, Baribault H, Schmidt C, Kemler R (1991) The structure of the gene coding for the mouse cell adhesion molecule uvomorulin. Nucleic Acids Res 19(23):6533–6539. https://doi.org/10.1093/nar/19.23.6533
Bussemakers MJ, Giroldi LA, van Bokhoven A, Schalken JA (1994) Transcriptional regulation of the human E-cadherin gene in human prostate cancer cell lines: characterization of the human E-cadherin gene promoter. Biochem Biophys Res Commun 203(2):1284–1290
Hennig G, Lowrick O, Birchmeier W, Behrens J (1996) Mechanisms identified in the transcriptional control of epithelial gene expression. J Biol Chem 271(1):595–602. https://doi.org/10.1074/jbc.271.1.595
Rodrigo I, Cato AC, Cano A (1999) Regulation of E-cadherin gene expression during tumor progression: the role of a new Ets-binding site and the E-pal element. Exp Cell Res 248(2):358–371. https://doi.org/10.1006/excr.1999.4438
Faraldo ML, Rodrigo I, Behrens J, Birchmeier W, Cano A (1997) Analysis of the E-cadherin and P-cadherin promoters in murine keratinocyte cell lines from different stages of mouse skin carcinogenesis. Mol Carcinog 20(1):33–47
Batsche E, Muchardt C, Behrens J, Hurst HC, Cremisi C (1998) RB and c-Myc activate expression of the E-cadherin gene in epithelial cells through interaction with transcription factor AP-2. Mol Cell Biol 18(7):3647–3658. https://doi.org/10.1128/mcb.18.7.3647
Schwartz B, Melnikova VO, Tellez C, Mourad-Zeidan A, Blehm K, Zhao YJ, McCarty M, Adam L, Bar-Eli M (2007) Loss of AP-2alpha results in deregulation of E-cadherin and MMP-9 and an increase in tumorigenicity of colon cancer cells in vivo. Oncogene 26(28):4049–4058. https://doi.org/10.1038/sj.onc.1210193
Leask A, Byrne C, Fuchs E (1991) Transcription factor AP2 and its role in epidermal-specific gene expression. Proc Natl Acad Sci U S A 88(18):7948–7952. https://doi.org/10.1073/pnas.88.18.7948
Choi I, Carey TS, Wilson CA, Knott JG (2012) Transcription factor AP-2gamma is a core regulator of tight junction biogenesis and cavity formation during mouse early embryogenesis. Development 139(24):4623–4632. https://doi.org/10.1242/dev.086645
Stemmler MP, Hecht A, Kemler R (2005) E-cadherin intron 2 contains cis-regulatory elements essential for gene expression. Development 132(5):965–976. https://doi.org/10.1242/dev.01662
Stemmler MP, Hecht A, Kinzel B, Kemler R (2003) Analysis of regulatory elements of E-cadherin with reporter gene constructs in transgenic mouse embryos. Dev Dyn 227(2):238–245. https://doi.org/10.1002/dvdy.10301
Jacobs J, Atkins M, Davie K, Imrichova H, Romanelli L, Christiaens V, Hulselmans G, Potier D, Wouters J, Taskiran II, Paciello G, Gonzalez-Blas CB, Koldere D, Aibar S, Halder G, Aerts S (2018) The transcription factor grainy head primes epithelial enhancers for spatiotemporal activation by displacing nucleosomes. Nat Genet 50(7):1011–1020. https://doi.org/10.1038/s41588-018-0140-x
Giroldi LA, Bringuier PP, de Weijert M, Jansen C, van Bokhoven A, Schalken JA (1997) Role of E boxes in the repression of E-cadherin expression. Biochem Biophys Res Commun 241(2):453–458. https://doi.org/10.1006/bbrc.1997.7831
Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, Baulida J, Garcia De Herreros A (2000) The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol 2(2):84–89. https://doi.org/10.1038/35000034
Cano A, Perez-Moreno MA, Rodrigo I, Locascio A, Blanco MJ, del Barrio MG, Portillo F, Nieto MA (2000) The transcription factor snail controls epithelial-mesenchymal transitions by repressing E-cadherin expression. Nat Cell Biol 2(2):76–83. https://doi.org/10.1038/35000025
Bolos V, Peinado H, Perez-Moreno MA, Fraga MF, Esteller M, Cano A (2003) The transcription factor slug represses E-cadherin expression and induces epithelial to mesenchymal transitions: a comparison with snail and E47 repressors. J Cell Sci 116(Pt 3):499–511
Hajra KM, Chen DY, Fearon ER (2002) The SLUG zinc-finger protein represses E-cadherin in breast cancer. Cancer Res 62(6):1613–1618
Eger A, Aigner K, Sonderegger S, Dampier B, Oehler S, Schreiber M, Berx G, Cano A, Beug H, Foisner R (2005) DeltaEF1 is a transcriptional repressor of E-cadherin and regulates epithelial plasticity in breast cancer cells. Oncogene 24(14):2375–2385. https://doi.org/10.1038/sj.onc.1208429
Grooteclaes ML, Frisch SM (2000) Evidence for a function of CtBP in epithelial gene regulation and anoikis. Oncogene 19(33):3823–3828. https://doi.org/10.1038/sj.onc.1203721
Comijn J, Berx G, Vermassen P, Verschueren K, van Grunsven L, Bruyneel E, Mareel M, Huylebroeck D, van Roy F (2001) The two-handed E box binding zinc finger protein SIP1 downregulates E-cadherin and induces invasion. Mol Cell 7(6):1267–1278
Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA (2004) Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117(7):927–939. https://doi.org/10.1016/j.cell.2004.06.006
Skrypek N, Goossens S, De Smedt E, Vandamme N, Berx G (2017) Epithelial-to-mesenchymal transition: epigenetic reprogramming driving cellular plasticity. Trends Genet 33(12):943–959. https://doi.org/10.1016/j.tig.2017.08.004
Dong C, Wu Y, Yao J, Wang Y, Yu Y, Rychahou PG, Evers BM, Zhou BP (2012) G9a interacts with snail and is critical for snail-mediated E-cadherin repression in human breast cancer. J Clin Invest 122(4):1469–1486. https://doi.org/10.1172/JCI57349
Lin T, Ponn A, Hu X, Law BK, Lu J (2010) Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition. Oncogene 29(35):4896–4904. https://doi.org/10.1038/onc.2010.234
Peinado H, Ballestar E, Esteller M, Cano A (2004) Snail mediates E-cadherin repression by the recruitment of the Sin3A/histone deacetylase 1 (HDAC1)/HDAC2 complex. Mol Cell Biol 24(1):306–319. https://doi.org/10.1128/mcb.24.1.306-319.2004
Herranz N, Pasini D, Diaz VM, Franci C, Gutierrez A, Dave N, Escriva M, Hernandez-Munoz I, Di Croce L, Helin K, Garcia de Herreros A, Peiro S (2008) Polycomb complex 2 is required for E-cadherin repression by the Snail1 transcription factor. Mol Cell Biol 28(15):4772–4781. https://doi.org/10.1128/MCB.00323-08
Tong ZT, Cai MY, Wang XG, Kong LL, Mai SJ, Liu YH, Zhang HB, Liao YJ, Zheng F, Zhu W, Liu TH, Bian XW, Guan XY, Lin MC, Zeng MS, Zeng YX, Kung HF, Xie D (2012) EZH2 supports nasopharyngeal carcinoma cell aggressiveness by forming a co-repressor complex with HDAC1/HDAC2 and snail to inhibit E-cadherin. Oncogene 31(5):583–594. https://doi.org/10.1038/onc.2011.254
Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, Mikulits W, Brabletz T, Strand D, Obrist P, Sommergruber W, Schweifer N, Wernitznig A, Beug H, Foisner R, Eger A (2007) The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene 26(49):6979–6988. https://doi.org/10.1038/sj.onc.1210508
Spaderna S, Schmalhofer O, Wahlbuhl M, Dimmler A, Bauer K, Sultan A, Hlubek F, Jung A, Strand D, Eger A, Kirchner T, Behrens J, Brabletz T (2008) The transcriptional repressor ZEB1 promotes metastasis and loss of cell polarity in cancer. Cancer Res 68(2):537–544. https://doi.org/10.1158/0008-5472.CAN-07-5682
Vandewalle C, Comijn J, De Craene B, Vermassen P, Bruyneel E, Andersen H, Tulchinsky E, Van Roy F, Berx G (2005) SIP1/ZEB2 induces EMT by repressing genes of different epithelial cell-cell junctions. Nucleic Acids Res 33(20):6566–6578. https://doi.org/10.1093/nar/gki965
Whiteman EL, Liu CJ, Fearon ER, Margolis B (2008) The transcription factor snail represses Crumbs3 expression and disrupts apico-basal polarity complexes. Oncogene 27(27):3875–3879. https://doi.org/10.1038/onc.2008.9
De Craene B, Gilbert B, Stove C, Bruyneel E, van Roy F, Berx G (2005) The transcription factor snail induces tumor cell invasion through modulation of the epithelial cell differentiation program. Cancer Res 65(14):6237–6244. https://doi.org/10.1158/0008-5472.CAN-04-3545
Ohkubo T, Ozawa M (2004) The transcription factor snail downregulates the tight junction components independently of E-cadherin downregulation. J Cell Sci 117(Pt 9):1675–1685. https://doi.org/10.1242/jcs.01004
Ikenouchi J, Matsuda M, Furuse M, Tsukita S (2003) Regulation of tight junctions during the epithelium-mesenchyme transition: direct repression of the gene expression of claudins/occludin by snail. J Cell Sci 116(Pt 10):1959–1967. https://doi.org/10.1242/jcs.00389
Martinez-Estrada OM, Culleres A, Soriano FX, Peinado H, Bolos V, Martinez FO, Reina M, Cano A, Fabre M, Vilaro S (2006) The transcription factors slug and snail act as repressors of Claudin-1 expression in epithelial cells. Biochem J 394(Pt 2):449–457. https://doi.org/10.1042/BJ20050591
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–428. https://doi.org/10.1038/nrc2131
Shamir ER, Pappalardo E, Jorgens DM, Coutinho K, Tsai WT, Aziz K, Auer M, Tran PT, Bader JS, Ewald AJ (2014) Twist1-induced dissemination preserves epithelial identity and requires E-cadherin. J Cell Biol 204(5):839–856. https://doi.org/10.1083/jcb.201306088
Chaffer CL, Weinberg RA (2011) A perspective on cancer cell metastasis. Science 331(6024):1559–1564. https://doi.org/10.1126/science.1203543
Brabletz T, Kalluri R, Nieto MA, Weinberg RA (2018) EMT in cancer. Nat Rev Cancer 18(2):128–134. https://doi.org/10.1038/nrc.2017.118
Campbell K (2018) Contribution of epithelial-mesenchymal transitions to organogenesis and cancer metastasis. Curr Opin Cell Biol 55:30–35. https://doi.org/10.1016/j.ceb.2018.06.008
Cheung KJ, Padmanaban V, Silvestri V, Schipper K, Cohen JD, Fairchild AN, Gorin MA, Verdone JE, Pienta KJ, Bader JS, Ewald AJ (2016) Polyclonal breast cancer metastases arise from collective dissemination of keratin 14-expressing tumor cell clusters. Proc Natl Acad Sci U S A 113(7):E854–E863. https://doi.org/10.1073/pnas.1508541113
Aceto N, Bardia A, Miyamoto DT, Donaldson MC, Wittner BS, Spencer JA, Yu M, Pely A, Engstrom A, Zhu H, Brannigan BW, Kapur R, Stott SL, Shioda T, Ramaswamy S, Ting DT, Lin CP, Toner M, Haber DA, Maheswaran S (2014) Circulating tumor cell clusters are oligoclonal precursors of breast cancer metastasis. Cell 158(5):1110–1122. https://doi.org/10.1016/j.cell.2014.07.013
Pyrgaki C, Liu A, Niswander L (2011) Grainyhead-like 2 regulates neural tube closure and adhesion molecule expression during neural fold fusion. Dev Biol 353(1):38–49. https://doi.org/10.1016/j.ydbio.2011.02.027
Werth M, Walentin K, Aue A, Schonheit J, Wuebken A, Pode-Shakked N, Vilianovitch L, Erdmann B, Dekel B, Bader M, Barasch J, Rosenbauer F, Luft FC, Schmidt-Ott KM (2010) The transcription factor grainyhead-like 2 regulates the molecular composition of the epithelial apical junctional complex. Development 137(22):3835–3845. https://doi.org/10.1242/dev.055483
Aue A, Hinze C, Walentin K, Ruffert J, Yurtdas Y, Werth M, Chen W, Rabien A, Kilic E, Schulzke JD, Schumann M, Schmidt-Ott KM (2015) A Grainyhead-like 2/Ovo-like 2 pathway regulates renal epithelial barrier function and lumen expansion. J Am Soc Nephrol 26(11):2704–2715. https://doi.org/10.1681/ASN.2014080759
Gao X, Vockley CM, Pauli F, Newberry KM, Xue Y, Randell SH, Reddy TE, Hogan BL (2013) Evidence for multiple roles for grainyhead-like 2 in the establishment and maintenance of human mucociliary airway epithelium.[corrected]. Proc Natl Acad Sci U S A 110(23):9356–9361. https://doi.org/10.1073/pnas.1307589110
Senga K, Mostov KE, Mitaka T, Miyajima A, Tanimizu N (2012) Grainyhead-like 2 regulates epithelial morphogenesis by establishing functional tight junctions through the organization of a molecular network among claudin3, claudin4, and Rab25. Mol Biol Cell 23(15):2845–2855. https://doi.org/10.1091/mbc.E12-02-0097
Mlacki M, Kikulska A, Krzywinska E, Pawlak M, Wilanowski T (2015) Recent discoveries concerning the involvement of transcription factors from the Grainyhead-like family in cancer. Exp Biol Med 240(11):1396–1401. https://doi.org/10.1177/1535370215588924
Cieply B, Riley P, Pifer PM, Widmeyer J, Addison JB, Ivanov AV, Denvir J, Frisch SM (2012) Suppression of the epithelial-mesenchymal transition by Grainyhead-like-2. Cancer Res 72(9):2440–2453. https://doi.org/10.1158/0008-5472.CAN-11-4038
Chung VY, Tan TZ, Tan M, Wong MK, Kuay KT, Yang Z, Ye J, Muller J, Koh CM, Guccione E, Thiery JP, Huang RY (2016) GRHL2-miR-200-ZEB1 maintains the epithelial status of ovarian cancer through transcriptional regulation and histone modification. Sci Rep 6:19943. https://doi.org/10.1038/srep19943
Nevil M, Bondra ER, Schulz KN, Kaplan T, Harrison MM (2017) Stable binding of the conserved transcription factor grainy head to its target genes throughout Drosophila melanogaster development. Genetics 205(2):605–620. https://doi.org/10.1534/genetics.116.195685
Boglev Y, Wilanowski T, Caddy J, Parekh V, Auden A, Darido C, Hislop NR, Cangkrama M, Ting SB, Jane SM (2011) The unique and cooperative roles of the grainy head-like transcription factors in epidermal development reflect unexpected target gene specificity. Dev Biol 349(2):512–522. https://doi.org/10.1016/j.ydbio.2010.11.011
Watanabe K, Villarreal-Ponce A, Sun P, Salmans ML, Fallahi M, Andersen B, Dai X (2014) Mammary morphogenesis and regeneration require the inhibition of EMT at terminal end buds by Ovol2 transcriptional repressor. Dev Cell 29(1):59–74. https://doi.org/10.1016/j.devcel.2014.03.006
Lee B, Villarreal-Ponce A, Fallahi M, Ovadia J, Sun P, Yu QC, Ito S, Sinha S, Nie Q, Dai X (2014) Transcriptional mechanisms link epithelial plasticity to adhesion and differentiation of epidermal progenitor cells. Dev Cell 29(1):47–58. https://doi.org/10.1016/j.devcel.2014.03.005
Kitazawa K, Hikichi T, Nakamura T, Mitsunaga K, Tanaka A, Nakamura M, Yamakawa T, Furukawa S, Takasaka M, Goshima N, Watanabe A, Okita K, Kawasaki S, Ueno M, Kinoshita S, Masui S (2016) OVOL2 maintains the transcriptional program of human corneal epithelium by suppressing epithelial-to-mesenchymal transition. Cell Rep 15(6):1359–1368. https://doi.org/10.1016/j.celrep.2016.04.020
Dai X, Schonbaum C, Degenstein L, Bai W, Mahowald A, Fuchs E (1998) The ovo gene required for cuticle formation and oogenesis in flies is involved in hair formation and spermatogenesis in mice. Genes Dev 12(21):3452–3463. https://doi.org/10.1101/gad.12.21.3452
Nair M, Bilanchone V, Ortt K, Sinha S, Dai X (2007) Ovol1 represses its own transcription by competing with transcription activator c-Myb and by recruiting histone deacetylase activity. Nucleic Acids Res 35(5):1687–1697. https://doi.org/10.1093/nar/gkl1141
Hong T, Watanabe K, Ta CH, Villarreal-Ponce A, Nie Q, Dai X (2015) An Ovol2-Zeb1 mutual inhibitory circuit governs bidirectional and multi-step transition between epithelial and mesenchymal states. PLoS Comput Biol 11(11):e1004569. https://doi.org/10.1371/journal.pcbi.1004569
Watanabe K, Liu Y, Noguchi S, Murray M, Chang JC, Kishima M, Nishimura H, Hashimoto K, Minoda A, Suzuki H (2019) OVOL2 induces mesenchymal-to-epithelial transition in fibroblasts and enhances cell-state reprogramming towards epithelial lineages. Sci Rep 9(1):6490. https://doi.org/10.1038/s41598-019-43021-z
Chakrabarti R, Hwang J, Andres Blanco M, Wei Y, Lukacisin M, Romano RA, Smalley K, Liu S, Yang Q, Ibrahim T, Mercatali L, Amadori D, Haffty BG, Sinha S, Kang Y (2012) Elf5 inhibits the epithelial-mesenchymal transition in mammary gland development and breast cancer metastasis by transcriptionally repressing Snail2. Nat Cell Biol 14(11):1212–1222. https://doi.org/10.1038/ncb2607
Yeung TL, Leung CS, Wong KK, Gutierrez-Hartmann A, Kwong J, Gershenson DM, Mok SC (2017) ELF3 is a negative regulator of epithelial-mesenchymal transition in ovarian cancer cells. Oncotarget 8(10):16951–16963. https://doi.org/10.18632/oncotarget.15208
Gondkar K, Patel K, Krishnappa S, Patil A, Nair B, Sundaram GM, Zea TT, Kumar P (2019) E74 like ETS transcription factor 3 (ELF3) is a negative regulator of epithelial- mesenchymal transition in bladder carcinoma. Cancer Biomark 25(2):223–232. https://doi.org/10.3233/CBM-190013
Ng AY, Waring P, Ristevski S, Wang C, Wilson T, Pritchard M, Hertzog P, Kola I (2002) Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology 122(5):1455–1466
Oliver JR, Kushwah R, Wu J, Pan J, Cutz E, Yeger H, Waddell TK, Hu J (2011) Elf3 plays a role in regulating bronchiolar epithelial repair kinetics following Clara cell-specific injury. Lab Invest 91(10):1514–1529. https://doi.org/10.1038/labinvest.2011.100
Albino D, Longoni N, Curti L, Mello-Grand M, Pinton S, Civenni G, Thalmann G, D'Ambrosio G, Sarti M, Sessa F, Chiorino G, Catapano CV, Carbone GM (2012) ESE3/EHF controls epithelial cell differentiation and its loss leads to prostate tumors with mesenchymal and stem-like features. Cancer Res 72(11):2889–2900. https://doi.org/10.1158/0008-5472.CAN-12-0212
Tymms MJ, Ng AY, Thomas RS, Schutte BC, Zhou J, Eyre HJ, Sutherland GR, Seth A, Rosenberg M, Papas T, Debouck C, Kola I (1997) A novel epithelial-expressed ETS gene, ELF3: human and murine cDNA sequences, murine genomic organization, human mapping to 1q32.2 and expression in tissues and cancer. Oncogene 15(20):2449–2462. https://doi.org/10.1038/sj.onc.1201427
Oliver JR, Kushwah R, Hu J (2012) Multiple roles of the epithelium-specific ETS transcription factor, ESE-1, in development and disease. Lab Invest 92(3):320–330. https://doi.org/10.1038/labinvest.2011.186
Laffin B, Wellberg E, Kwak HI, Burghardt RC, Metz RP, Gustafson T, Schedin P, Porter WW (2008) Loss of singleminded-2s in the mouse mammary gland induces an epithelial-mesenchymal transition associated with up-regulation of slug and matrix metalloprotease 2. Mol Cell Biol 28(6):1936–1946. https://doi.org/10.1128/MCB.01701-07
Acloque H, Ocana OH, Matheu A, Rizzoti K, Wise C, Lovell-Badge R, Nieto MA (2011) Reciprocal repression between Sox3 and snail transcription factors defines embryonic territories at gastrulation. Dev Cell 21(3):546–558. https://doi.org/10.1016/j.devcel.2011.07.005
Barrallo-Gimeno A, Nieto MA (2005) The snail genes as inducers of cell movement and survival: implications in development and cancer. Development 132(14):3151–3161. https://doi.org/10.1242/dev.01907
Traylor-Knowles N, Hansen U, Dubuc TQ, Martindale MQ, Kaufman L, Finnerty JR (2010) The evolutionary diversification of LSF and Grainyhead transcription factors preceded the radiation of basal animal lineages. BMC Evol Biol 10:101. https://doi.org/10.1186/1471-2148-10-101
Barrallo-Gimeno A, Nieto MA (2009) Evolutionary history of the snail/scratch superfamily. Trends Genet 25(6):248–252. https://doi.org/10.1016/j.tig.2009.04.001
Pattabiraman DR, Bierie B, Kober KI, Thiru P, Krall JA, Zill C, Reinhardt F, Tam WL, Weinberg RA (2016) Activation of PKA leads to mesenchymal-to-epithelial transition and loss of tumor-initiating ability. Science 351(6277):aad3680. https://doi.org/10.1126/science.aad3680
Park SM, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22(7):894–907. https://doi.org/10.1101/gad.1640608
Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10(5):593–601. https://doi.org/10.1038/ncb1722
Bracken CP, Gregory PA, Kolesnikoff N, Bert AG, Wang J, Shannon MF, Goodall GJ (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 68(19):7846–7854. https://doi.org/10.1158/0008-5472.CAN-08-1942
Burk U, Schubert J, Wellner U, Schmalhofer O, Vincan E, Spaderna S, Brabletz T (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9(6):582–589. https://doi.org/10.1038/embor.2008.74
Abba ML, Patil N, Leupold JH, Allgayer H (2016) MicroRNA regulation of epithelial to mesenchymal transition. J Clin Med 5(1). https://doi.org/10.3390/jcm5010008
Zhou BP, Deng J, Xia W, Xu J, Li YM, Gunduz M, Hung MC (2004) Dual regulation of snail by GSK-3beta-mediated phosphorylation in control of epithelial-mesenchymal transition. Nat Cell Biol 6(10):931–940. https://doi.org/10.1038/ncb1173
Peinado H, Del Carmen Iglesias-de la Cruz M, Olmeda D, Csiszar K, Fong KS, Vega S, Nieto MA, Cano A, Portillo F (2005) A molecular role for lysyl oxidase-like 2 enzyme in snail regulation and tumor progression. EMBO J 24(19):3446–3458. https://doi.org/10.1038/sj.emboj.7600781
Jung HY, Fattet L, Tsai JH, Kajimoto T, Chang Q, Newton AC, Yang J (2019) Apical-basal polarity inhibits epithelial-mesenchymal transition and tumour metastasis by PAR-complex-mediated SNAI1 degradation. Nat Cell Biol 21(3):359–371. https://doi.org/10.1038/s41556-019-0291-8
Weng M, Wieschaus E (2016) Myosin-dependent remodeling of adherens junctions protects junctions from snail-dependent disassembly. J Cell Biol 212(2):219–229. https://doi.org/10.1083/jcb.201508056
Campbell K, Casanova J, Skaer H (2010) Mesenchymal-to-epithelial transition of intercalating cells in drosophila renal tubules depends on polarity cues from epithelial neighbours. Mech Dev 127(7-8):345–357. https://doi.org/10.1016/j.mod.2010.04.002
Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M (1987) Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature 329(6137):341–343. https://doi.org/10.1038/329341a0
Mege RM, Matsuzaki F, Gallin WJ, Goldberg JI, Cunningham BA, Edelman GM (1988) Construction of epithelioid sheets by transfection of mouse sarcoma cells with cDNAs for chicken cell adhesion molecules. Proc Natl Acad Sci U S A 85(19):7274–7278. https://doi.org/10.1073/pnas.85.19.7274
Auersperg N, Pan J, Grove BD, Peterson T, Fisher J, Maines-Bandiera S, Somasiri A, Roskelley CD (1999) E-cadherin induces mesenchymal-to-epithelial transition in human ovarian surface epithelium. Proc Natl Acad Sci U S A 96(11):6249–6254. https://doi.org/10.1073/pnas.96.11.6249
McCrea PD, Gottardi CJ (2016) Beyond beta-catenin: prospects for a larger catenin network in the nucleus. Nat Rev Mol Cell Biol 17(1):55–64. https://doi.org/10.1038/nrm.2015.3
Conacci-Sorrell M, Simcha I, Ben-Yedidia T, Blechman J, Savagner P, Ben-Ze'ev A (2003) Autoregulation of E-cadherin expression by cadherin-cadherin interactions: the roles of beta-catenin signaling, slug, and MAPK. J Cell Biol 163(4):847–857. https://doi.org/10.1083/jcb.200308162
Pece S, Gutkind JS (2000) Signaling from E-cadherins to the MAPK pathway by the recruitment and activation of epidermal growth factor receptors upon cell-cell contact formation. J Biol Chem 275(52):41227–41233. https://doi.org/10.1074/jbc.M006578200
Rubsam M, Mertz AF, Kubo A, Marg S, Jungst C, Goranci-Buzhala G, Schauss AC, Horsley V, Dufresne ER, Moser M, Ziegler W, Amagai M, Wickstrom SA, Niessen CM (2017) E-cadherin integrates mechanotransduction and EGFR signaling to control junctional tissue polarization and tight junction positioning. Nat Commun 8(1):1250. https://doi.org/10.1038/s41467-017-01170-7
Li R, Liang J, Ni S, Zhou T, Qing X, Li H, He W, Chen J, Li F, Zhuang Q, Qin B, Xu J, Li W, Yang J, Gan Y, Qin D, Feng S, Song H, Yang D, Zhang B, Zeng L, Lai L, Esteban MA, Pei D (2010) A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell 7(1):51–63. https://doi.org/10.1016/j.stem.2010.04.014
Samavarchi-Tehrani P, Golipour A, David L, Sung HK, Beyer TA, Datti A, Woltjen K, Nagy A, Wrana JL (2010) Functional genomics reveals a BMP-driven mesenchymal-to-epithelial transition in the initiation of somatic cell reprogramming. Cell Stem Cell 7(1):64–77. https://doi.org/10.1016/j.stem.2010.04.015
Li Q, Hutchins AP, Chen Y, Li S, Shan Y, Liao B, Zheng D, Shi X, Li Y, Chan WY, Pan G, Wei S, Shu X, Pei D (2017) A sequential EMT-MET mechanism drives the differentiation of human embryonic stem cells towards hepatocytes. Nat Commun 8:15166. https://doi.org/10.1038/ncomms15166
Ward C, Volpe G, Cauchy P, Ptasinska A, Almaghrabi R, Blakemore D, Nafria M, Kestner D, Frampton J, Murphy G, Buganim Y, Kaji K, Garcia P (2018) Fine-tuning Mybl2 is required for proper mesenchymal-to-epithelial transition during somatic reprogramming. Cell Rep 24(6):1496–1511. e1498. https://doi.org/10.1016/j.celrep.2018.07.026
Downing TL, Soto J, Morez C, Houssin T, Fritz A, Yuan F, Chu J, Patel S, Schaffer DV, Li S (2013) Biophysical regulation of epigenetic state and cell reprogramming. Nat Mater 12(12):1154–1162. https://doi.org/10.1038/nmat3777
Fatehullah A, Tan SH, Barker N (2016) Organoids as an in vitro model of human development and disease. Nat Cell Biol 18(3):246–254. https://doi.org/10.1038/ncb3312
Shaw TJ, Martin P (2016) Wound repair: a showcase for cell plasticity and migration. Curr Opin Cell Biol 42:29–37. https://doi.org/10.1016/j.ceb.2016.04.001
Acknowledgments
Funding: Work in the Röper lab is supported by the Medical Research Council (file reference number U105178780 and BSF30).
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2021 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Ng-Blichfeldt, JP., Röper, K. (2021). Mesenchymal-to-Epithelial Transitions in Development and Cancer. In: Campbell, K., Theveneau, E. (eds) The Epithelial-to Mesenchymal Transition. Methods in Molecular Biology, vol 2179. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0779-4_7
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0779-4_7
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0778-7
Online ISBN: 978-1-0716-0779-4
eBook Packages: Springer Protocols