Abstract
With increased globalisation and homogenisation, the maintenance of genetic integrity in local populations of agriculturally important species is of increasing concern. The western honeybee (Apis mellifera) provides an interesting perspective as it is both managed and wild, with a large native range and much larger introduced range. We employed a newly created 95 single nucleotide polymorphism (SNP) test to characterise the genetic ancestry of the Australian commercial and feral honeybee populations. We found that most individuals were hybrids of mainly Western and Eastern European ancestry. Introductions of bees from North Africa are known from the historical record, and we show here the presence of alleles of African ancestry in some Australian bees, at levels comparable to those seen in the commercial populations of European-derived bees in North America.
Similar content being viewed by others
1 Introduction
Maintaining genetic diversity in domesticated animals is of increasing concern (Hall 2004; Ajmone-Marsan & GLOBALDIV Consortium 2010; Groeneveld et al. 2010; Lenstra et al. 2012). In general, domestication leads to a loss of genetic diversity, although this is not evident in the Western honeybee Apis mellifera (Whitfield et al. 2006; Harpur et al. 2012, 2014; Wallberg et al. 2014). Nevertheless there are legitimate concerns over the loss of genetic diversity and increasing homogenisation in A. mellifera, both between and within populations (De la Rua et al. 2009; Dietemann et al. 2009; Meixner et al. 2010; De la Rua et al. 2013; Harpur et al. 2013), and the maintenance of genetic diversity within colonies, which increases honey production, nest homeostasis and disease resistance (Palmer & Oldroyd 2003; Tarpy 2003; Tarpy & Seeley 2006; Mattila & Seeley 2007; Oldroyd & Fewell 2007; Seeley & Tarpy 2007; Tarpy et al. 2013).
Though its native range was originally confined to Africa, Europe and the Middle East (Seeley 1985; Winston 1987; Ruttner 1988), the distribution of A. mellifera now extends over most of the world. The honeybee was spread by humans for its value in honey and wax production and crop pollination. It has been estimated that 84 % of world crops depend on insect pollinators, including honeybees (Klein et al. 2007). In Australia, 35 industries and 65 % of agricultural production are wholly or partially dependent on honeybee pollination (Keogh et al. 2010).
There is considerable controversy over the number of evolutionary lineages and subspecies within A. mellifera, and which subspecies belong to which lineage (Ruttner 1988). Four major lineages (clades) are generally recognised—African (A), Eastern European (C), Western European (M) and Middle Eastern (O) (Ruttner 1988; Garnery et al. 1992; Franck et al. 2000; Whitfield et al. 2006; Harpur et al. 2014; Wallberg et al. 2014) although up to two additional lineages have been suggested (Franck et al. 2001; Alburaki et al. 2011; Alburaki et al. 2013). The commonly used subspecies by commercial beekeepers are A. m. ligustica and A. m. carnica from the Eastern European lineage.
A number of subspecies have been introduced to Australia. The earliest imports were from Western Europe—A. m. mellifera (and perhaps A. m. iberiensis (iberica) Chapman et al. 2008). Descendants of these bees quickly spread throughout the country (Coleman 1956; Ruttner 1976; Weatherhead 1986; Oldroyd et al. 1995). Several decades later, A. m. ligustica (early 1860s) and A. m. carnica (from 1890s) were introduced from Eastern Europe as well as A. m. caucasica from the O lineage (from 1880s) (Hopkins 1886; Eckert 1958; Weatherhead 1986; Woodward 1993; Gulliford 2005; Rhodes 2011), along with A. m. intermissa and A. m. lamarckii from Africa in the late nineteenth century (Weatherhead 1986; Gulliford 2005; Rhodes 2011), A. m. cypria from Cyprus between 1896 and 1952 (Goodacre 1935; Gulliford 2005) and A. m. syriaca from Syria in the late nineteenth century (Gulliford 2005; Rhodes 2011) and perhaps others not known from the historical record. Today, the favoured subspecies in Australia are ‘Italian’ or ‘Ligurian’ (A. m. ligustica), ‘Carniolan’ (A. m. carnica) and ‘Caucasian’ (A. m. caucasica). An online search for ‘Australian bee breeders’ yields 13 breeders, ten who propagate A. m. ligustica, four A. m. caucasica and one A. m. carnica, while two did not specify a subspecies.
Genetic studies of Australian bees have identified signatures of the European lineages only (Oldroyd et al. 1993; 1995; Koulianos & Crozier 1996, 1997; Chapman et al. 2008; Oxley & Oldroyd 2009). The bees from Kangaroo Island, 20 km off the South Australian coast, are still reputed to be the most ‘pure’ A. m. ligustica strain in the world, despite carrying mitochondria of A. m. mellifera origin (Oldroyd et al. 1993; Koulianos & Crozier 1996, 1997) and allozymes at frequencies associated with A. m. siciliana (Oldroyd et al. 1993). Bees from the Tasmanian highlands have previously been characterised as being of A. m. mellifera origin (Oldroyd et al. 1995; Koulianos & Crozier 1996, 1997), while other, warmer, Tasmania regions have a mix of A. m. ligustica and some A. m. mellifera (Oldroyd et al. 1995; Koulianos & Crozier 1997). Mitotypes consistent with A. m. ligustica (Western Australia [WA], New South Wales [NSW], Victoria [VIC], Tasmania [TAS]), A. m. caucasica (NSW) and A. m. mellifera (WA, Kangaroo Island [KI], TAS, VIC, NSW) have been found in Australia (Koulianos & Crozier 1997). Mitochondrial sequences associated with A. m. ligustica (commercial population ) and A. m. iberiensis (commercial and feral population) have been identified in WA (Chapman et al. 2008) while those of A. m. ligustica, A. m. carnica and A. m. caucasica, and possibly A. m. macedonica, have been identified in commercial colonies from the other states (Oxley & Oldroyd 2009).
In this study, we examine both feral and commercial Australian honeybees with 95 single nucleotide polymorphism (SNP) markers newly created to differentiate between the African, Western European and Eastern European lineages (Chapman et al. 2015) in order to obtain a genetic profile of Australian honeybees.
2 Methods
We obtained samples from 102 colonies from the commercial population (Figure 1; Supplementary Information) and 104 feral (unmanaged) samples from both colonies and drone congregation areas (Figure 2; Supplementary Information). DNA was extracted from one individual per colony with phenol-chloroform isoamyl alcohol (Sambrook et al. 1989) and typed at 95 SNPs (Chapman et al. 2015) using the Sequenom MassARRAY MALDI-TOF system (Sequenom, CA, USA). The 95 SNPs were chosen on the basis of high pairwise F ST values between the African, Western European and Eastern European lineages (Harpur et al. 2014) or because they were hypothesised to be under selection (Whitfield et al. 2006), had minor allele frequencies greater than 5 % and more than two thirds of genotypes successfully typed (Chapman et al. 2015). SNPs were unlinked; on average, SNPs were separated by 1,734,863 base pairs and the closest SNPs were 45,945 base pairs apart (Chapman et al. 2015). Haploid data were coded as missing data at one allele for each SNP marker (Pritchard et al. 2010). Ancestry was assigned to individuals based on reference populations from the Western European (M; A. m. mellifera and A. m. iberiensis; n = 13), Eastern European (C; A. m. carnica and A. m. ligustica; n = 86) and African (A; A. m. scutellata; n = 128) lineages (Chapman et al. 2015) using STRUCTURE (v2.3.4 Pritchard et al. 2000) with three ancestral lineages assumed (but not given a priori) and a burn-in phase of 50,000 iterations followed by 100,000 iterations with admixture assumed and uncorrelated allele frequencies.
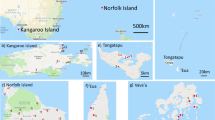
Geographical distribution of sampled individuals from the commercial Australian population with the proportion of ancestry from Eastern European (C; yellow), Western European (M; black) and African (A; pink) lineages. 1 Samples from Norfolk Island, 1412 km east of mainland Australia. 2 Samples from the Western Australian Better Bees breeding programme (Chapman et al. 2008) for which no location is given.
Geographical distribution of sampled individuals from the feral Australian population depicting the proportion of ancestry from Eastern Europe (C; yellow), Western Europe (M; black) and Africa (A; pink). Sampling locations outlined in blue were sampled by collecting drones at drone congregation areas.
3 Results
The commercial and feral populations of Australia carry a similar (low) proportion of alleles from the African lineage (3.83 versus 3.97 %). The commercial population carries more Eastern European alleles (69.36 versus 57.39 %) and fewer Western European alleles (26.81 versus 38.64 %) than the feral population, although the difference in ancestry proportions from the three lineages between the two populations was not statistically significant (χ 2 2 = 3.271, P = 0.195; Figure 3).
The bees from the highlands in the centre of Tasmania have a high proportion of Western European alleles (commercial, 86.3–92.0 %, Figure 1; feral, 81.1–90.0 %, Figure 2). In contrast, bees collected from elsewhere in Tasmania (commercial, 11.7–30.9 %; Figure 1) and Australia (commercial, 1.0–53.1 %; feral, 10.9–77.5 %) have a much lower frequency of alleles of Western European origin. A number of feral samples (seven NSW, three VIC, one SA and seven WA) had a high proportion (>50 %) of Western European alleles (Figure 2), while only two (VIC) commercial individuals had more than 50 % alleles of Western European ancestry (Figure 1). Few individuals could be considered ‘pure’ in either population. In the commercial population, only three individuals had greater than 90 % Eastern European alleles (two NSW and one SA) and another three had greater than 90 % Western European alleles (all from the Tasmanian highlands; Figure 1), while in the feral population, one individual carried 90 % Western European alleles (from the Tasmania highlands; Figure 2).
Most individuals had few alleles that are present at high frequency in the African lineage reference population A. m. scutellata (Figures 1 and 2). In the commercial population, 80.77 % of individuals carried fewer than 5 % African alleles, while in the feral population, it was 75.49 % (Figure 4). Only 6.73 % of commercial samples carried greater than 15 % African alleles, while in the feral population, it was 4.90 % (Figure 4).
4 Discussion
Using a SNP panel designed to differentiate between the African, Eastern European and Western European lineages (Chapman et al. 2015), we found that the commercial and feral honeybee populations of Australia are hybrids of mainly Eastern European and Western European ancestry. While the feral population generally carries more Western European M alleles than the commercial population (38.64 versus 26.81 %), the difference is not statistically significant. This is expected as the first reported introduction of honeybees into Australia were of A. m. mellifera from Western Europe, which were allowed to go wild and spread quickly across the country (Ruttner 1976; Weatherhead 1986; Oldroyd et al. 1995), and thus, feral populations have repeatedly been found to be of predominantly Western European origin (Oldroyd et al. 1995; Koulianos & Crozier 1997; Chapman et al. 2008). It is noteworthy that these alleles have persisted in the feral population despite continuous input of Eastern European lineage alleles from the commercial population for more than 100 years. This may suggest that the feral population is largely independent of the commercial population—as has previously been noted in Western Australia (Chapman et al. 2008). Alternatively, selection may be acting on the unmanaged feral population, such that genes with a selective advantage derived from the Western European lineage are maintained in the feral population, making them more adapted to the local environment (Harpur et al. 2014). Such a pattern has been found in the Africanized population from Brazil and USA, where some alleles from the Western European lineage are retained but those from the Eastern European lineage tend to be lost (Schneider et al. 2004; Harrison et al. 2006; Whitfield et al. 2006; Harpur et al. 2012; Wallberg et al. 2014). These Western European alleles may not be retained in the commercial Australian population as beekeepers select for productivity and other attributes that may be at a selective disadvantage in unmanaged populations. Selection may also be acting to retain the African alleles given their persistence and the limited historical introduction of bees from North Africa.
For the first time, we found a genetic signature associated with reference samples from Africa in Australia. The historical record suggests that there were a small number of introductions of bees from North Africa to Australia in the late nineteenth and early twentieth centuries (Weatherhead 1986; Gulliford 2005; Rhodes 2011). The African reference samples are A. m. scutellata, which has not been imported to Australia, and the identification of alleles associated with Africa does not imply that A. m. scutellata was ever imported. There were also some limited introductions from the Middle East (Goodacre 1935; Gulliford 2005), and more extensively of A. m. caucasia which has been identified as both Eastern European and Middle Eastern. The Middle Eastern lineage (O) derived from the Eastern European (C) lineage, but has had more recent (in evolutionary time) hybridization with the African (A) lineage (Wallberg et al. 2014). Thus, it is likely that some of the African alleles detected were introduced through importations from the Middle East. A similar proportion of African ancestry has also been shown in commercial populations of the USA, Canada and Europe (Schiff et al. 1994; Whitfield et al. 2006; Wallberg et al. 2014; Chapman et al. 2015). It is clear that these bees are not ‘Africanized’ as populations of Africanized bees from Brazil carry 80.33 % African alleles on average when genotyped with this SNP panel, while those from America carry 62.42 % on average (Chapman et al. 2015).
Our data and the historical record of imports of honeybees into Australia indicate that like the North American population, the Australian population is likely to be highly hybridised and genetically diverse (Harpur et al. 2012, 2013). While Australian bee breeders tend to advertise queens of a particular subspecies, most often this is not true because commercial queens mate with a proportion of feral drones or any other strains that are in the area. Genetic diversity provides opportunities for selection of desirable traits and may benefit individual colonies via direct heterosis (Cale & Gowen 1956; Oldroyd et al. 1985; Oldroyd & Moran 1987; Oldroyd et al. 1987) and social heterosis wherein mixtures of genotypes within colonies can improve the task allocation system and colony performance (Oldroyd et al. 1992; Jones et al. 2004; Chapman et al. 2007; Mattila & Seeley 2007; Oldroyd & Fewell 2007) and disease resistance (Palmer & Oldroyd 2003; Tarpy 2003; Tarpy & Seeley 2006; Seeley & Tarpy 2007). Thus, the hybrid origin of bees introduced to Australia is likely to promote the health and productivity of Australian honeybees; if a limited number of colonies from a single site had been introduced, then we are likely to have experienced problems with inbreeding. Concerns over inbreeding have been addressed in previous studies (Chapman et al. 2008; Oxley & Oldroyd 2009).
Within the native range of the honeybee, there are strong arguments for conservation of local ecotypes as sources of genetic diversity, including local adaptations to environmental conditions and pathogens (Strange et al. 2007; De la Rua et al. 2009; Meixner et al. 2010; Oleska et al. 2011; Chavez-Galarza et al. 2013; De la Rua et al. 2013; Harpur et al. 2013; Pinto et al. 2014). The cooperation of local beekeepers in keeping only the desired strain is central to the success of such conservation programmes (Jensen et al. 2005; Jensen & Pedersen 2005). The SNP panel used in this study provides a new tool to measure the success of such conservation programmes and to identify the ancestry of introduced populations.
References
Ajmone-Marsan, P., GLOBALDIV Consortium (2010) A global view of livestock biodiversity and conservation - GLOBALDIV. Anim. Genet. 41(Supplementary 1), 1–5
Alburaki, M., Bertrand, B., Legout, H., Moulin, S., Alburaki, A., Sheppard, W.S., Garnery, L. (2013) A fifth major genetic group among honeybees revealed in Syria. BMC Genet. 14, 117
Alburaki, M., Moulin, S., Legout, H., Alburaki, A., Garnery, L. (2011) Mitochondrial structure of Eastern honey bee populations from Syria, Lebanon and Iraq. Apidologie 42, 628–641
Cale, G.H., Gowen, J.W. (1956) Heterosis in the honey bee (Apis mellifera L.). Genetics 41, 292–303
Chapman, N.C., Harpur, B.A., Lim, J., Rinderer, T.E., Allsopp, M., Zayed, A., Oldroyd, B.P. (2015) A SNP test to identify Africanized honeybees via proportion of 'African' ancestry. Mol. Ecol. Resour. doi:10.1111/1755-0998.12411
Chapman, N.C., Lim, J., Oldroyd, B.P. (2008) Population genetics of commercial and feral honey bees in Western Australia. J. Econ. Entomol. 101, 272–277
Chapman, N.C., Oldroyd, B.P., Hughes, W.O.H. (2007) Differential responses of honeybee (Apis mellifera) patrilines to changes in stimuli for the generalist tasks of nursing and foraging. Behav. Ecol. Sociobio. 61, 1185–1194
Chavez-Galarza, J., Henriques, D., Johnston, J.S., Azevedo, J.C., Patton, J.C., Munoz, I., de la Rua, P., Pinto, M.A. (2013) Signatures of selection in the Iberian honey bee (Apis mellifera iberiensis) revealed by a genome scan analysis of single nucleotide polymorphisms. Mol. Ecol. 22, 5890–5907
Coleman, R.S. (1956) Beekeeping in Western Australia - some historical notes. J. Agr. West Aust. 5, 6
De la Rua, P., Jaffe, R., Dall'Olio, R., Munzos, I., Serrana, J. (2009) Biodiversity, conservation and current threats to European honeybees. Apidologie 40, 263–284
De la Rua, P., Jaffe, R., Munoz, I., Serrano, J., Moritz, R.F.A., Kraus, F.B. (2013) Conserving genetic diversity in the honeybee: Comments on Harpur et al. (2012). Mol. Ecol. 22, 3208–3210
Dietemann, V., Pirk, C.W.W., Crewe, R. (2009) Is there a need for conservation of honeybees in Africa? Apidologie 40, 285–295
Eckert, J.E. (1958) The Kangaroo Island Ligurian bees. Gleanings in Bee Culture 86(660–663), 722–725
Franck, P., Garnery, L., Loiseau, A., Oldroyd, B.P., Hepburn, H.R., Solignac, M., Cornuet, J.M. (2001) Genetic diversity of the honeybee in Africa: microsatellite and mitochondrial data. Heredity 86, 420–430
Franck, P., Garnery, L., Solignac, M., Cornuet, J.M. (2000) Molecular confirmation of a fourth lineage in honeybees from the Near East. Apidologie 31, 167–180
Garnery, L., Cornuet, J.-M., Solignac, M. (1992) Evolutionary history of the honey bee Apis mellifera inferred from mitochondrial DNA analysis. Mol. Ecol. 1, 145–154
Goodacre W.A. (1935) The beginner in bee culture. In: Farmer's Bulletin, p. 91. Department of Agriculture New South Wales, Sydney, Australia.
Groeneveld, L.F., Lenstra, J.A., Eding, H., Toro, M.A., Scherf, B., Pilling, D., Negrini, R., Finlay, E.K., Jianlin, H., Groeneveld, E., Weigend, S., GLOBALDIV Consortium (2010) Genetic diversity in farm animals - a review. Anim. Genet. 41(Supplement 1), 6–31
Gulliford, R. (2005) Beekeeping in Australia. The Australasian Beekeeper, Rutherford, Australia
Hall, S.J.G. (2004) Livestock Biodiversity. Genetic Resources for the Farming of the Future. Blackwell Science, Oxford, UK
Harpur, B.A., Kent, C.F., Molodtsova, D., Lebon, J.M.D., Alqarni, A.S., Owayss, A.A., Zayed, A. (2014) Population genomics of the honey bee reveals strong signatures of positive selection on worker traits. Proc. Natl. Acad. Sci. USA 111, 2614–2619
Harpur, B.A., Minaei, S., Kent, C.F., Zayed, A. (2012) Management increases genetic diversity of honey bees via admixture. Mol. Ecol. 21, 4414–4421
Harpur, B.A., Minaei, S., Kent, C.F., Zayed, A. (2013) Admixture increases diversity in managed honey bees: Reply to De la Rua etal. (2013). Mol. Ecol. 22, 3211–3215
Harrison, J.F., Fewell, J.H., Anderson, K.E., Loper, G.M. (2006) Environmental physiology of the invasion of the Americas by Africanized honeybees. Integr. Comp. Biol. 46, 1110–1122
Hopkins, I. (1886) Illustrated Australasian Bee Manual and Complete Guide to Modern Bee Culture in the Southern Hemisphere, 3rd edn. Issac Hopkins, Auckland, New Zealand
Jensen, A.B., Palmer, K.A., Boomsma, J.J., Pedersen, B.V. (2005) Varying degrees of Apis mellifera ligustica introgression in protected populations of the back honeybee, Apis mellifera mellifera, in northwest Europe. Mol. Ecol. 14, 93–106
Jensen, A.B., Pedersen, B.V. (2005) Honeybee conservation: A case story from Laeso Island, Denmark. In: Lodesani, M., Costa, C. (eds.) Beekeeping and conserving biodiversity of honeybees, pp. 142–164. Northern Bee Books, Hebden Bridge UK
Jones, J.C., Myerscough, M.R., Graham, S., Oldroyd, B.P. (2004) Honey bee nest thermoregulation: Diversity promotes stability. Science 305, 402–404
Keogh, R.C., Robinson, A.P.W., Mullins, I.J. (2010) Pollination Aware: The Real Value of Pollination in Australia. Rural Industries Research and Development Corporation, Canberra, Australia
Klein, A.M., Vaissiere, B.E., Cane, J.H., Steffan-Dewenter, I., Cunningham, S.A., Kremen, C., Tscharntke, T. (2007) Importance of pollinators in changing landscapes for world crops. P. Roy. Soc. B. Biol. Sci. 274, 303–313
Koulianos, S., Crozier, R. (1996) Mitochondrial DNA sequence data provides further evidence that the honeybees of Kangaroo Island, Australia are of hybrid origin. Apidologie 27, 165–174
Koulianos, S., Crozier, R. (1997) Mitochondrial sequence characterisation of Australian commercial and feral honeybee strains, Apis mellifera L. (Hymenoptera: Apidae), in the context of the species worldwide. Aust. J. Entomol. 36, 359–363
Lenstra, J.A., Groeneveld, L.F., Eding, H., Kantanen, J., Williams, J.L., Taberlet, P., Nicolazzi, E.L., Solkner, J., Simianer, H., Ciani, E., Garcia, J.R., Bruford, M.W., Ajmone-Marsan, P., Weigend, S. (2012) Molecular tools and analytical approaches for the characterization of farm animal genetic diversity. Anim. Genet. 43, 483–502
Mattila, H.R., Seeley, T.D. (2007) Genetic diversity in honey bee colonies enhances productivity and fitness. Science 317, 362
Meixner, M.D., Costa, C., Kryger, P., Hatjina, F., Bouga, M., Ivanova, E., Buchler, R. (2010) Conserving diversity and vitality for honey bee breeding. J. Apic. Res. 49, 85–92
Oldroyd, B.P., Cornuet, J.M., Rowe, D., Rinderer, T.E., Crozier, R.H. (1995) Racial admixture of Apis mellifera in Tasmania, Australia - similarities and differences with natural hybrid zones in Europe. Heredity 74, 315–325
Oldroyd, B.P., Fewell, J.H. (2007) Genetic diversity promotes homeostasis in insect colonies. Trends Ecol. Evol. 22, 408–413
Oldroyd, B.P., Moran, C. (1987) Additive and heterotic genetic effects in the haplo-diploid honeybee Apis mellifera. Aust. J. Bio. Sci. 40, 57–63
Oldroyd, B.P., Moran, C., Nicholas, F.W. (1985) Diallele crosses of honeybees 1. A genetic-analysis of honey production using a fixed effects model. J. Apic. Res. 24, 243–249
Oldroyd, B.P., Moran, C., Nicholas, F.W. (1987) Diallele crosses of honeybees 2. A note presenting an estimate of the heritability of honey production under Australian conditions. Aust. J. Agric. Res. 38, 651–654
Oldroyd, B.P., Rinderer, T.E., Harbo, J.R., Buco, S.M. (1992) Effects of intracolonial genetic diversity of honey bee (Hymenoptera, Apidae) colony performance. Ann. Entomol. Soc. Am. 85, 335–343
Oldroyd, B.P., Sheppard, W.S., Stelzer, J.A. (1993) Genetic characterization of the bees of Kangaroo Island, South Australia. J. Apic. Res. 31, 141–148
Oleska, A., Chybicki, I., Tofilski, A., Burczyk, J. (2011) Nuclear and mitochondrial patterns of introgression into native dark bees (Apis mellifera mellifera) in Poland. J. Apic. Res. 50, 116–129
Oxley, P.R., Oldroyd, B.P. (2009) Mitochondrial sequencing reveals five separate origins of 'Black' Apis mellifera (Hymenoptera: Apidae) in Eastern Australian commercial colonies. J. Econ. Entomol. 102, 480–484
Palmer, K.A., Oldroyd, B.P. (2003) Evidence for intra-colonial genetic variance in resistance to American foulbrood of honey bees (Apis mellifera): Further support for the parasite/pathogen hypothesis for the evolution of polyandry. Naturwissenschaften 90, 265–268
Pinto, M.A., Henriques, D., Chaves-Galarza, J., Kryger, P., Garnery, L., Van der Zee, M., Dahle, B., Soland-Reckeweg, G., de la Rua, P., Dall'Olio, R., Carreck, N.L., Johnson, J.S. (2014) Genetic integrity of the Dark European honey bee (Apis mellifera mellifera) from protected populations: A genome-wide assessment using SNPs and mtDNA sequence data. J. Apic. Res. 53, 269–278
Pritchard, J.K., Stephens, M., Donnelly, P. (2000) Inference of population structure using multilocus genotype data. Genetics 155, 945–959
Pritchard J.K., Wen X., Falush D. (2010) Documentation for structure software: Version 2.3, p. 7, http://pritchardlab.stanford.edu/structure_software/release_versions/v2.3.4/structure_doc.pdf.
Rhodes J.W. (2011) Quality of commercially reared queen and drone honey bees (Apis mellifera L.) in eastern Australia, University of Western Sydney, Australia.
Ruttner, F. (1976) Isolated populations of honeybees in Australia. J. Apic. Res. 15, 97–104
Ruttner, F. (1988) Biogeography and Taxonomy of Honeybees. Springer, Berlin, Germany
Sambrook, J., Fritsch, E.F., Maniatis, T. (1989) Molecular cloning, 2nd edn. Cold Spring Harbor Laboratory Press, New York, USA
Schiff, N.M., Sheppard, W.S., Loper, G.M., Shimanuki, H. (1994) Genetic diversity of feral honey bee (Hymenoptera: Apidae) populations in the southern United States. Ann. Entomol. Soc. Am. 87, 842–848
Schneider, S.S., DeGrandi-Hoffman, G., Smith, D.R. (2004) The African honey bee: factors contributing to a successful biological invasion. Annu. Rev. Entomol. 49, 351–376
Seeley, T.D. (1985) Honeybee Ecology: A Study of Adaptation in Social Life. Princeton University Press, Princeton, USA
Seeley, T.D., Tarpy, D.R. (2007) Queen promiscuity lowers disease within honeybee colonies. P. Roy. Soc. B Bio. Sci. 274, 67–72
Strange, J.P., Garnery, L., Sheppard, W.S. (2007) Persistence of the Landes ecotype of Apis mellifera mellifera in southwest France: confirmation of a locally adaptive annual brood cycle trait. Apidologie 38, 259–267
Tarpy, D.R. (2003) Genetic diversity within honeybee colonies prevents severe infections and promotes colony growth. P. Roy. Soc. B Biol. Sci. 270, 99–103
Tarpy, D.R., Seeley, T.D. (2006) Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs. monandrous queens. Naturwissenschaften 93, 195–199
Tarpy, D.R., van Engelsdorp, D., Pettis, J.S. (2013) Genetic diversity affects colony survivorship in commercial honey bee colonies. Naturwissenschaften 100, 723–728
Wallberg, A., Han, F., Wellhagen, G., Dahle, B., Kawata, M., Haddad, N., Simoes, Z.L.P., Allsopp, M.H., Kandemir, I., de la Rua, P., Pirk, C.W., Webster, M.T. (2014) A worldwide survey of genome sequence variation provides insight into the evolutionary history of the honeybee Apis mellifera. Nature Genet. 48, 1081–1088
Weatherhead T. (1986) Boxes to Bar Hives: Beekeeping History of Queensland. International Colour Productions.
Whitfield, C.W., Behura, S.K., Berlocher, S.H., Clark, A.G., Johnston, J.S., Sheppard, W.S., Smith, D.R., Suarez, A.V., Weaver, D.B., Tsutsui, N.D. (2006) Thrice out of Africa: ancient and recent expansions of the honey bee, Apis mellifera. Science 314, 642–645
Winston, M.L. (1987) The Biology of the Honey Bee. Harvard University Press, Cambridge
Woodward, D. (1993) Ligurian bees. Am. Bee J. 133, 124–125
Acknowledgments
This project was supported by grants from Rural Industries Research and Development Corporation PRJ-007774 Australia (BPO), a Natural Sciences and Engineering Research Council Discover grant (AZ) and an Ontario Ministry of Research and Innovations Early Researcher Award (AZ). We thank the Australian Department of Agriculture, Fisheries and Forestry, Plant Health Australia, Biosecurity Australia and many beekeepers for contributing samples. We thank the team at Australian Cancer Research Fund at the Garvan Institute for use of their facilities.
Author contributions
BPO, AZ and NCC designed the experiment. NCC and JL extracted DNA. NCC performed genotyping and data analysis. AZ and BAH provided SNPs. AZ and BAH provided data. BPO, NCC, MHA and TER provided samples. NCC and BPO wrote the paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Marina Meixner
Origines hybrides des abeille australiennes (Apis mellifera)
Australie / apiculture / polymorphisme de nucléotide simple / sous-espèce / hybridation
Australische Honigbienen (Apis mellifera) sind gemischter Herkunft
Australien / Bienenhaltung / Single Nucleotide Polymorphismus / Unterarten der Honigbiene / Hybriden
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 122 kb)
Rights and permissions
About this article
Cite this article
Chapman, N.C., Harpur, B.A., Lim, J. et al. Hybrid origins of Australian honeybees (Apis mellifera). Apidologie 47, 26–34 (2016). https://doi.org/10.1007/s13592-015-0371-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13592-015-0371-0