Abstract
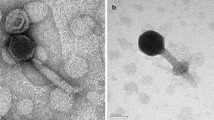
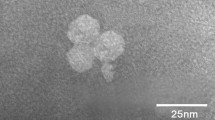
A cyanophage strain and its host Synechococcus were isolated from the East China Sea. The host Synechococcus sp. SJ01 was characterized by its 16S rRNA, ITS, and psbA gene sequences as well as by its morphological appearance and pigmentation. The cyanophage, strain S-SJ2, was able to cause a lytic infection of the coastal Synechococcus. TEM of negative-stained specimens showed that the phage isolate has an isometric head with a diameter of 68 nm and a long tail with a length of 280 nm. The cyanophage-Synechococcus system from the East China Sea shares many properties with other marine cyanophage-Synechococcus systems worldwide.
Similar content being viewed by others
References
Angly F E, Felts B, Breitbart M, Salamon P, Edwards R A, Carlson C, Chan A M, Haynes M, Kelley S, Liu H, Mahaffy J M, Mueller J E, Nulton J, Olson R, Parsons R, Rayhawk S, Suttle C A, Rohwer F. 2006. The marine viromes of four oceanic regions. PLoS Biol. 4: e368.
Becker W E. 1994. Microalgae biotechnology. In: Studies on Biotechnology. (Eds.): Sir James Braddily, N. H. Carr, I. J., Higgins, W. G. Potters. University Press, Cambridge.
Bench S R, Hanson T E, Williamson K E, Ghosh D, Radosovich M, Wang K, Wommack K E.2007. Metagenomic characterization of Chesapeake Bay virioplankton. Appl Environ Microbiol. 73: 7629–7641.
Chen F, Suttle C A, Short S M. 1996. Genetic diversity in marine algal virus communities as revealed by sequence analysis of DNA polymerase genes. Appl Environ Microbiol. 62: 2869–2874.
Fuller N J, Marie D, Partensky F, Vaulot D, Post A F, Scanlan D J. 2003. Clade-specific 16S ribosomal DNA oligonucleotides reveal the predominance of a single marine Synechococcus clade throughout a stratified watercolumn in the Red Sea. Appl Environ Microbiol. 69: 2430–2443.
Huang S, Wang K, Jiao N. 2012. Genome sequences of siphoviruses infecting marine Synechococcus unveil a diverse cyanophage group and extensive phage-host genetic exchanges. Environ Microbiol. 14:540–558.
Li W K W. 1994. Primary production of prochlorophytes, cyanobacteria, and eukaryotic ultraphytoplankton: measurements from flow cytometric sorting. Limnol Oceanogr. 39: 169–175.
Lindell D, Sullivan M B, Zackary I J. 2004. Transfer of photosynthesis genes to and from Prochlorococcus viruses. PNAS.101.30.11013-11018.
Lu J, Chen F, Hodson R E. 2001. Distribution, Isolation, Host Specificity, and Diversity of Cyanophages Infecting Marine Synechococcus spp. Appl Environ Microbiol. 67: 3285–3290.
Mann N H.2003. Phages of the marine cyanobacterial picophytoplankton. FEMS Microbiol Rev. 27: 17–34.
Marston M F, Sallee J L. 2003. Genetic diversity and temporal variation in the cyanophage community infesting marine Synechococcus species in Rhode Island’s coastal waters. Appl Environ Microbiol. 69: 4639–4647.
Neilan B A, Jacobs D, Del D T, Blackall L L, Hawkins P R, Cox P T, Goodman A E. 1997. rRNA sequences and evolutionary relationships among toxic and nontoxic cyanobacteria of the genus Microcystis. IJSB. 47: 693–697.
Ong L J, Glazer A N, Waterbury J B.1984. An unusual phycoerythrin from a marine cyanobacterium. Science. 224: 80–83.
Palacios D M, Bograd S J, Mendelssohn R, Schwing F B.2004. Long-term and seasonal trends in stratification in the California Current, 1950–1993. J Geophys Res. 109: C10016.
Partensky F, Blanchot J, Vaulot D. 1999. Differential distribution and ecology of Prochlorococcus and Synechococcus in oceanic waters: a review. Bull Inst Oceanogr. 19: 457–475.
Prangishvili D, Forterre P, Garrett R A. 2006. Viruses of the Archaea: A unifying view. Nat Rev Microbiol. 4: 837–848.
Rippka R. 1988. Isolation and purification of cyanobacteria. In: Methods in Enzymology. 167: 1–7.
Rocap G, Distel D L, Waterbury J B, Chisholm S W. 2002. Resolution of prochlorococcus and synechococcus ecotypes by using 16S-23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol. 68: 1180–1191.
Sabehi G, Shaulou L, Silver D H, Yanai I, Harel A, Lindell D. 2011. A novel lineage of myoviruses infecting cyanobacteria is widespread in the oceans. PNAS. 109:2037–2042.
Scanlan, D J, West N J. 2002. Molecular ecology of the marine cyanobacterial genera Prochlorococcus and Synechococcus. FEMS Microbiol Ecol. 40: 1–12.
Singh P, Singh S S, Srivastava A, Singh A, Mishra A K. 2012. Structural, functional and molecular basis of cyanophage-cyanobacterial interactions and its significance. Afr Biotechnol. 11: 2591–2608.
Sullivan M B, Coleman M L, Quinlivan V. 2008. Portal protein diversity and phage ecology. Environ Microbiol.10: 2810–2823.
Suttle C A, Chan A M. 1993. Marine cyanophages infecting oceanic and coastal strains of Synechococcus: abundance, morphyology, cross-infectivity and growth characteristics. Mar Ecol Prog Ser. 92: 99–109.
Suttle C A, Chan A M. 1994. Dynamics and distribution of cyanophages and their effect on marine Synechococcus spp. Appl Environ Microbiol. 60: 3167–3174.
Throdson J. 1969. Flagellates of Norwegian coastal waters. Nytt Mag Bot. 6(3-4): 161–216.
Tillett D, Neilan B A. 2000. Rapid nucleic acid isolation from cultured and environmental cyanobacteria: novel techniques based on xanthogenate. J Phycol. 36: 251–258.
Van Etten J L, Lane L C, Dunigan DD. 2010. DNA viruses: The really big ones (giruses). Ann Rev Microbiol. 64: 83–99.
Vera T, Brian P. 2009. Temporal variation of Synechococcus clades at a coastal Pacific Ocean monitoring site. ISME. 3: 903–915.
Vidaver A K, Koski R K, Van Etten J L. 1973. Bacteriophage Φ6: a lipid-containing virus of Pseudomonas phaseolicola. J Virol. 11: 799–805.
Wang K, Wommack K E, Chen F. 2011. Abundance and distribution of Synechococcus spp. and cyanophages in the Chesapeake Bay. Appl Environ Microbiol. l77: 7459–7468.
Waterbury J B, Valois F W. 1993. Resistance to co-occurring phages enables marine Synechococcus communities to coexist with cyanophages abundant in seawater. Appl Environ Microbiol. 59: 3393–3399.
Waterbury J B, Watson S W, Valois F W, Franks D G. 1986. Biological and ecological characterization of the marine unicellular cyanobacteria Synechococcus. Can Bull Fish Aquat Sci. 214: 71–120.
Weitz J S, Poisot T, Meyer J R, Flores C O, Valverde S, Sullivan MB, Hochberg ME. 2013. Phage-bacteria infection networks. Trends Microbiol. 21: 82–91.
Wilson W H, Joint I R, Carr N G, Mann N H. 1993. Isolation and molecular characterization of five marine cyanophages propagated on Synechococcus sp. strain WH7803. Appl Environ Microbiol. 59: 3736–3743.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, Y., Xu, M., Zhao, Y. et al. The first isolation of a cyanophage-Synechococcus system from the East China Sea. Virol. Sin. 28, 260–265 (2013). https://doi.org/10.1007/s12250-013-3333-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12250-013-3333-6