Abstract
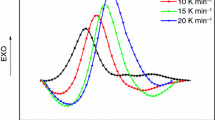
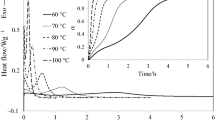
A new homologous series of curing agents (LCECAn) containing 4,4′-biphenyl and n-methylene units (n = 2, 4, 6) were successfully synthesized. The curing behaviors of a commercial diglycidyl ether of bisphenol-A epoxy (E-51) and 4,4′-bis(2,3-epoxypropoxy)biphenyl (LCE) by using LCECAn as the curing agent have been investigated by differential scanning calorimetry (DSC), respectively. The Ozawa equation was applied to the curing kinetics based upon the dynamic DSC data, and the isothermal DSC data were fitted using an autocatalytic curing model. The glass transition temperatures (T g) of the cured epoxy systems were determined by DSC upon the second heating, and the thermal decomposition temperatures (T d) were obtained by thermogravimetric (TG) analyses. The results show that the number of methylene units in LCECAn has little influence on the curing temperatures of E-51/LCECAn and LCE/LCECAn systems. In addition, the activation energies obtained by the dynamic method proved to be larger than those by the isothermal method. Furthermore, both the T g and T d of the cured E-51/LCECAn systems and LCE/LCECAn systems decreased with the increase in the number of methylene units in LCECAn.









Similar content being viewed by others
References
Zhang B-L, Tang G-L, Shi K-Y, You Y-C, Du Z-J, Yang J-F, et al. A study on properties of epoxy resin toughened by functionalized polymer containing rigid, rod-like moiety. Eur Polym J. 2000;36:205–13.
He S, Shi K, Bai J, Zhang Z, Li L, Du Z, et al. Studies on the properties of epoxy resins modified with chain-extended ureas. Polymer. 2001;42:9641–7.
He SJ, Shi KY, Guo XZ, Du ZJ, Zhang BL. Properties of methyltetrahydrophthalic anhydride-cured epoxy resin modified with MITU. Polym Adv Technol. 2009;20:130–4.
Zhang B-L, Tang G-L, Shi K-Y, You Y-C, Du Z-J, Huang J-F. A study on the properties of epoxy resin toughened by a liquid crystal-type oligomer. J Appl Polym Sci. 1999;71:177–84.
Ma S, Liu W, Su Q, Liu Y. Studies on the thermal properties of epoxy resins modified with two kinds of silanes. J Macromol Sci: Phys. 2010;49:43–56.
Villanueva M, Fraga I, Rodríguez-Añón J, Proupín-Castiñeiras J. Study of the influence of a reactive diluent on the rheological properties of an epoxy-diamine system. J Therm Anal Calorim. 2009;98:521–5.
Villanueva M, Martín-Iglesias J, Rodríguez-Añón J, Proupín-Castiñeiras J. Thermal study of an epoxy system DGEBA (n = 0)/mXDA modified with POSS. J Therm Anal Calorim. 2009;96:575–82.
López J, Rico M, Montero B, Díez J, Ramírez C. Polymer blends based on an epoxy-amine thermoset and a thermoplastic. J Therm Anal Calorim. 2009;95:369–76.
Yousefi A, Lafleur PG, Gauvin R. Kinetic studies of thermoset cure reactions: a review. Polym Compos. 1997;18:157–68.
Kamal MR. Thermoset characterization for moldability analysis. Polym Eng Sci. 1974;14:231–9.
Batch GL, Macosko CW. Kinetic model for crosslinking free radical polymerization including diffusion limitations. J Appl Polym Sci. 1992;44:1711–29.
Patel PS, Shah PP, Patel SR. Differential scanning calorimetry investigation of curing of bisphenolfurfural resins. Polym Eng Sci. 1986;26:1186–90.
Vinnik R, Roznyatovsky V. Kinetic method by using calorimetry to mechanism of epoxy-amine cure reaction. J Therm Anal Calorim. 2003;73:807–17.
Liu X, Sheng X, Lee J, Kessler M. Isothermal cure characterization of dicyclopentadiene. J Therm Anal Calorim. 2007;89:453–7.
Kissinger HE. Reaction kinetics in differential thermal analysis. Anal Chem. 1957;29:1702–6.
Ozawa T. Kinetic analysis of derivative curves in thermal analysis. J Therm Anal Calorim. 1970;2:301–24.
Kamal MR, Sourour S. Kinetics and thermal characterization of thermoset cure. Polym Eng Sci. 1973;13:59–64.
Mormann W, Bröcher M. “Liquid crystalline” thermosets from 4,4′-bis(2, 3-epoxypropoxy)biphenyl and aromatic diamines. Macromol Chem Phys. 1996;197:1841–51.
Ando M, Uryu T. Synthesis of polymer materials by low energy electron beam. XIII. Structure and properties of EB-cured polymers of bifunctional monomer with biphenyl moiety as mesogenic group. J Polym Sci A: Polym Chem. 1990;28:2575–84.
Giamberjni M, Amendola E, Carfagna C. Liquid crystalline epoxy thermosets. Mol Cryst Liq Cryst. 1995;266:9–22.
Barton JM. Monitoring the curing reaction of an aromatic amine/epoxide resin system by differential scanning calorimetry (DSC): determination and significance of the activation energy. Makromol Chem. 1973;171:247–51.
Punchaipetch P, Ambrogi V, Giamberini M, Brostow W, Carfagna C, D’Souza NA. Epoxy + liquid crystalline epoxy coreacted networks: I. Synthesis and curing kinetics. Polymer. 2001;42:2067–75.
Prime RB. Differential scanning calorimetry of the epoxy cure reaction. Polym Eng Sci. 1973;13:365–71.
Riccardi CC, Dupuy J, Williams RJJ. A simple model to explain the complex kinetic behavior of epoxy/anhydride systems. J Polym Sci B: Polym Phys. 1999;37:2799–805.
Peyser P, Bascom WD. Kinetics of epoxy resin polymerization using differential scanning calorimetry. J Appl Polym Sci. 1977;21:2359–73.
González-Romero VM, Casillas N. Isothermal and temperature programmed kinetic studies of thermosets. Polym Eng Sci. 1989;29:295–301.
Khanna U, Chanda M. Kinetics of anhydride curing of isophthalic diglycidyl ester using differential scanning calorimetry. J Appl Polym Sci. 1993;49:319–29.
Hale A, Macosko CW, Bair HE. Glass transition temperature as a function of conversion in thermosetting polymers. Macromolecules. 1991;24:2610–21.
Mathew AP, Packirisamy S, Thomas S. Studies on the thermal stability of natural rubber/polystyrene interpenetrating polymer networks: thermogravimetric analysis. Polym Degrad Stab. 2001;72:423–39.
Gupta A, Singhal R, Nagpal AK. Reactive blends of epoxy resin (DGEBA) crosslinked by anionically polymerized polycaprolactam: process of epoxy cure and kinetics of decomposition. J Appl Polym Sci. 2004;92:687–97.
Acknowledgements
Financial support provided by the National Natural Science Foundation of China (No. 50673042), and the Doctoral Discipline Foundation of Ministry of Education of China (No. 20070055015) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Guo, Q., Huang, Y., Zhang, YY. et al. Curing behavior of epoxy resins with a series of novel curing agents containing 4,4′-biphenyl and varying methylene units. J Therm Anal Calorim 102, 915–922 (2010). https://doi.org/10.1007/s10973-010-0764-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-010-0764-2