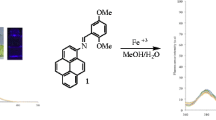
Abstract
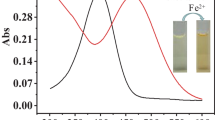
A new and efficient chemodosimeter for ferric ions has been developed. The visual and fluorescent behaviors of the compound toward various metal ions were investigated: ferric ions are distinguished from other cations by selective color change and unusual fluorescence enhancement in mixed aqueous solution. Fluorescence microscopy experiments showed that this receptor is effective for detection of Fe3+ in vitro, developing a good image of the biological organelles. The sensing mechanism is shown to involve a hydrolysis process.











Similar content being viewed by others
References
de Silva AP, Gunaratne HQN, Gunnlaugsson T, Huxley AJM, McCoy CP, Rademacher JT, Rice TE (1997) Signaling recognition events with fluorescent sensors and switches. Chem Rev 97(5):1515–1566
Desvergne J-P, Czarnik AW (1997) Chemosensors of ion and molecule recognition. Springer Science & Business Media
Lin W, Long L, Yuan L, Cao Z, Feng J (2009) A novel ratiometric fluorescent Fe3+ sensor based on a phenanthroimidazole chromophore. Anal Chim Acta 634(2):262–266
Valeur B, Leray I (2000) Design principles of fluorescent molecular sensors for cation recognition. Coord Chem Rev 205(1):3–40
Zhang M, Gao Y, Li M, Yu M, Li F, Li L, Zhu M, Zhang J, Yi T, Huang C (2007) A selective turn-on fluorescent sensor for FeIII and application to bioimaging. Tetrahedron Lett 48(21):3709–3712
Klaassen CD (2013) Casarett and Doull's toxicology: the basic science of poisons. McGraw-Hill, New York
D’Autréaux B, Tucker NP, Dixon R, Spiro S (2005) A non-haem iron centre in the transcription factor NorR senses nitric oxide. Nature 437(7059):769–772
Lee J-W, Helmann JD (2006) The PerR transcription factor senses H2O2 by metal-catalysed histidine oxidation. Nature 440(7082):363–367
Bharath S, Hsu M, Kaur D, Rajagopalan S, Andersen JK (2002) Glutathione, iron and Parkinson’s disease. Biochem Pharmacol 64(5):1037–1048
Honda K, Casadesus G, Petersen RB, Perry G, Smith MA (2004) Oxidative stress and redox‐active iron in Alzheimer’s Disease. Ann N Y Acad Sci 1012(1):179–182
Li Z, Yu M, Zhang L, Yu M, Liu J, Wei L, Zhang H (2010) A “switching on” fluorescent chemodosimeter of selectivity to Zn2+ and its application to MCF-7 cells. Chem Commun 46(38):7169–7171
Li Z, Zhang L, Wang L, Guo Y, Cai L, Yu M, Wei L (2011) Highly sensitive and selective fluorescent sensor for Zn 2+/Cu 2+ and new approach for sensing Cu 2+ by central metal displacement. Chem Commun 47(20):5798–5800
Jung HJ, Singh N, Lee DY, Jang DO (2010) Single sensor for multiple analytes: chromogenic detection of I− and fluorescent detection of Fe 3+. Tetrahedron Lett 51(30):3962–3965
Praveen L, Reddy M, Varma RL (2010) Dansyl-styrylquinoline conjugate as divalent iron sensor. Tetrahedron Lett 51(50):6626–6629
Kumar M, Kumar R, Bhalla V (2010) Optical chemosensor for Ag+, Fe3+, and cysteine: information processing at molecular level. Org Lett 13(3):366–369
Zhang X, Shiraishi Y, Hirai T (2007) A new rhodamine-based fluorescent chemosensor for transition metal cations synthesized by one-step facile condensation. Tetrahedron Lett 48(31):5455–5459
Li Z-X, Zhang L-F, Zhao W-Y, Li X-Y, Guo Y-K, Yu M-M, Liu J-X (2011) Fluoranthene-based pyridine as fluorescent chemosensor for Fe 3+. Inorg Chem Commun 14(10):1656–1658
Smanmoo S, Nasomphan W, Tangboriboonrat P (2011) Highly selective fluorescent chemosensor for Fe 3+ imaging in living cells. Inorg Chem Commun 14(2):351–354
Ouchetto H, Dias M, Mornet R, Lesuisse E, Camadro J-M (2005) A new route to trihydroxamate-containing artificial siderophores and synthesis of a new fluorescent probe. Bioorg Med Chem 13(5):1799–1803
Xiang Y, Tong A (2006) A new rhodamine-based chemosensor exhibiting selective FeIII-amplified fluorescence. Org Lett 8(8):1549–1552
Ghosh K, Rathi S, Kushwaha R (2013) Sensing of Fe (III) ion via turn-on fluorescence by fluorescence probes derived from 1-naphthylamine. Tetrahedron Lett 54(48):6460–6463
Quang DT, Kim JS (2010) Fluoro-and chromogenic chemodosimeters for heavy metal ion detection in solution and biospecimens. Chem Rev 110(10):6280–6301
Du J, Hu M, Fan J, Peng X (2012) Fluorescent chemodosimeters using “mild” chemical events for the detection of small anions and cations in biological and environmental media. Chem Soc Rev 41(12):4511–4535
Liu Z-H, Devaraj S, Yang C-R, Yen Y-P (2012) A new selective chromogenic and fluorogenic sensor for citrate ion. Sensors Actuators B Chem 174:555–562
Tsui Y-K, Devaraj S, Yen Y-P (2012) Azo dyes featuring with nitrobenzoxadiazole (NBD) unit: a new selective chromogenic and fluorogenic sensor for cyanide ion. Sensors Actuators B Chem 161(1):510–519
Bhorge YR, Tsai H-T, Huang K-F, Pape AJ, Janaki SN, Yen Y-P (2014) A new pyrene-based Schiff-base: a selective colorimetric and fluorescent chemosensor for detection of Cu (II) and Fe (III). Spectrochim Acta A Mol Biomol Spectrosc 130:7–12
Devaraj S, Tsui Y-k, Chiang C-Y, Yen Y-P (2012) A new dual functional sensor: highly selective colorimetric chemosensor for Fe 3+ and fluorescent sensor for Mg 2+. Spectrochim Acta A Mol Biomol Spectrosc 96:594–599
Lin C-Y, Huang K-F, Yen Y-P (2013) A new selective colorimetric and fluorescent chemodosimeter for based on hydrolysis of Schiff base. Spectrochim Acta A Mol Biomol Spectrosc 115:552–558
Araujo P (2009) Key aspects of analytical method validation and linearity evaluation. J Chromatogr B 877(23):2224–2234
Liu J, Lu Y (2007) Rational design of “turn-on” allosteric DNAzyme Catalytic beacons for aqueous mercury ions with ultrahigh sensitivity and selectivity. Angew Chem Int Ed 46(40):7587–7590
Ono A, Togashi H (2004) Highly selective oligonucleotide‐based sensor for mercury (II) in aqueous solutions. Angew Chem Int Ed 43(33):4300–4302
Acknowledgments
We thank the Ministry of Science and Technology, Taiwan, R.O.C., for the financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, YZ., Bhorge, Y.R., Pape, A.J. et al. A New Schiff Base Chemodosimeter for Fluorescent Imaging of Ferric Ions in Living Cells. J Fluoresc 25, 1331–1337 (2015). https://doi.org/10.1007/s10895-015-1622-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-015-1622-1