Abstract
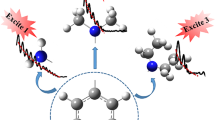
Pyrene-1-carboxy acid has a pK of 4 in the ground state, and a pK of 8 in the excited state. Fluorescence spectra of the acid and base forms are presented as a function of solvent and temperature. Ab initio quantum calculations indicate that the bond between the ring system and the carboxyl group has aromatic character that becomes stronger upon excitation. This stabilization helps to account for the increase in pK upon excitation.










Similar content being viewed by others
References
Weller A (1961) Fast reactions of excited molecules. Prog React Kinet 1:187–214
Ireland JF, Wyatt PAH (1976) Acid-base properties of electronically excited states of organic molecules. In: Gold V, Bethell D (eds) Advances in physical organic chemistry. London, Academic
Shimoni E, Nachliel E, Gutman M (1993) Gaugement of the inner space of the apomyoglobin’s heme binding site by a single free diffusing proton. II. Interaction with a bulk proton. Biophys J 64:480–483
Gepshtein R, Leiderman P, Huppert D, Project E, Nachliel E, Gutman M (2006) Proton antenna effect of the gamma-cyclodextrin outer surface, measured by excited state proton transfer. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys 110:26354–26364
Roche CJ, Guo F, Friedman JM (2006) Molecular level probing of preferential hydration and its modulation by osmolytes through the use of pyranine complexed to hemoglobin. J Biol Chem 281:38757–38768
Loken MR, Hayes JW, Gohlke JR, Brand L (1972) Excited-state proton transfer as a biological probe. Determination of rate constants by means of nanosecond fluorometry. Biochemistry 11:4779–4786
Rayner DM, Krajcarski, DT, Szabo AG (1978) Excited state acid-base equilibrium of tyrosine. Can J Chem 56:1238–1245
Martynov IY, Demyashkevich AB, Uzhinov BM, Kuz’min MG (1977) Proton transfer reactions in the excited electronic states of aromatic molecules. Russ Chem Rev Uspekhi Khimii 46:3–31.
Pines E, Huppert D, Gutman M, Nachliel E, Fishman M (1986) The pOH jump: determination of deprotonation rates of water by 6-methoxyquinoline and acridine. J Phys Chem 90:6366–6370
Zelent B, Vanderkooi JM, Coleman RG, Gryczynski I, Gryczynski Z (2006) Protonation of excited state pyrene-1-carboxylate by phosphate and organic acids in aqueous solution studied by fluorescence spectroscopy. Biophys J 91(10):3864–3871
Weller A (1952) Quantitative Untersuchungen der Fluoreszenzumwandlung bei Naphtholen. Z Elektrochem 56:662–668
Agmon N (2005) Elementary steps in excited-state proton transfer. J Phys Chem A 109:13–35
Agmon W, Rettig N, Groth C (2002) Electronic determinants of photoacidity in cyanonaphthols. J Am Chem Soc 124:1089–1096
Frisch M, Trucks G, Schlegel H, Scuseria G, Robb M, Cheeseman J, Zakrzewski G, Montgomery J, Stratmann R Jr., Buran J, Dapprich S, Millam J, Daniels A, Kudin K, Strain M, Farkas O, Tomasi J, Barone V, Cossi M, Cammi R, Mennucci B, Pomelli C, Adamo C, Clifford S, Ochterski J, Petersson G, Ayala P, Cui Q, Morokuma K, Salvador P, Dannenberg J, Malick D, Rabuck A, Raghavachari K, Foresman J, Cioslowski J, Ortiz J, Baboul A, Stefanov B, Liu G, Liashenko A, Piskorz P, Komaromi I, Gomperts R, Martin R, Fox D, Keith T, Al-Laham M, Peng C, Nanayakkara A, Challacombe M, Gill P, Johnson B, Chen W, Wong M, Andres J, Gonzalez C, Head-Gordon M, Replogle E, Pople J (1998) Gaussian 98. Gaussian, Pittsburgh, PA
Binning RC Jr, Curtiss LA (1990) Compact contracted basis sets for third-row atoms: gallium-krypton. J Comp Chem 11(10):1206–1216
Hariharan PC, Pople J (1973) Influence of polarization functions on MO hydrogenation energies. Theor Chim Acta 28(3):213–222
Hariharan PC, Pople J (1974) Accuracy of AHn equilibrium geometries by single determinant molecular orbital theory. Mol Phys 27(1):209–214
Hehre WJ, Ditchfield R, Pople J (1972) Self-consistent molecular orbital methods. XII. Further extensions of Gaussian-type basis sets for use in molecular orbital studies of organic molecules. J Chem Phys 56(5):2257–2261
Gelndening ED, Reed AE, Carpenter JE, WF NBO Version 3.1.
Vanderkooi JM, Callis JB (1974) Pyrene. A probe of lateral diffusion in the hydrophobic region of membranes. Biochemistry 13:4000–4006
Badea MG, Brand L (1978) Time-resolved fluorescence measurements. Methods Enzymol 61:378–425
Vander Donckt E, Dramaix R, Nasielski J, Vogels C (1969) Photochemistry of aromatic compounds. 1. Acid-base properties of singlet and triplet excited states of pyrene derivatives and aza-aromatic compounds. Trans Faraday Soc 65:3258–3263
Dierksen M, Grimme S (2004) Density functional calculations of the vibronic structure of electronic absorption spectra. J Chem Phys 120:3544–3554
Prabhu NV, Dalosto SD, Sharp KA, Wright WW, Vanderkooi JM (2002) Optical spectra of Fe(II) cytochrome c interpreted using molecular dynamics simulations and quantum mechanical calculations. J Phys Chem B 106:5561–5571
Acknowledgements
This work was supported by NIH grant PO1 GM48130 and fellowship grant NIH F31 NSO53399 to NVN.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nucci, N.V., Zelent, B. & Vanderkooi, J.M. Pyrene-1-Carboxylate in Water and Glycerol Solutions: Origin of the Change of pK Upon Excitation. J Fluoresc 18, 41–49 (2008). https://doi.org/10.1007/s10895-007-0233-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10895-007-0233-x