Abstract
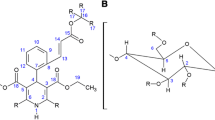
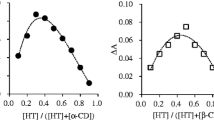
Complexation of ebastine (EB) with hydroxypropyl and methyl-β-cyclodextrin (HP-β-CD and Me-β-CD) was studied in aqueous solutions and in the solid state. The formation of inclusion complexes in aqueous solutions was analysed by the solubility method. The assays were designed using low CD concentrations compared with the solubility of these derivatives in order to avoid non-inclusion phenomena and to obtain a linear increase in EB solubility as a function of CD concentration. The values of complexation efficiency for HP-β-CD and Me-β-CD were 1.9 × 10−2 and 2.1 × 10−2, respectively. It seems that the non polar character of the methyl moiety slightly favoured complexation. In relation to solid state complexation, 1:1 EB:CD systems were prepared by kneading, and by heating a drug-CD mixture at 90 ºC. They were analysed using X ray diffraction analysis by comparison with their respective physical mixtures. A complex with a characteristic diffraction pattern similar to that of the channel structure of β-CD was formed with Me-β-CD in 1:1 melted and 1:2 EB:CD kneaded systems. Complexation with HP-β-CD was not clearly evidenced because only a slight reduction of drug crystallinity was detected. Finally, the loading of EB in two β-CD polymers cross-linked with epichlorohydrin yielded 7.3 and 7.7 mg of EB/g polymer respectively.



Similar content being viewed by others
References
Dahan, A., Miller, J.M., Amidon, L.: Prediction of solubility and permeability class membership: provisional BCS classification of the world’s top oral drugs. AAPS. J. 11, 740–746 (2009)
Gerebtzoff, G., Seelig, A.: In silico prediction of blood-brain barrier permeation using the calculated molecular cross-sectional area as main parameter. J. Chem. Inf. Model 46, 2638–2650 (2006)
Loftsson, T., Brewster, M.E., Másson, M.: Role of cyclodextrins in improving oral drug delivery. Am. J. Drug Deliv. 2, 261–275 (2004)
Brewster, M.E., Loftsson, T.: Cyclodextrins as pharmaceutical solubilizers. Adv. Drug Deliv. Rev. 59, 645–666 (2007)
Szejtli, J., Osa, T. (eds.): Comprehensive Supramolecular Chemistry: Cyclodextrins, vol. 3. Pergamon, Oxford (1996)
Uekama, K.: Design and evaluation of cyclodextrin-based drug formulation. Chem. Pharm. Bull. 52, 900–915 (2004)
van de Manakker, F., Vermonden, T., van Nostrum, C.F., Hennink, W.E.: Cyclodextrin-based polymeric materials: synthesis, properties and pharmaceutical/biomedical applications. Biomacromolecules 10, 3157–3175 (2009)
García-Zubiri, I.X., González-Gaitano, G., Isasi, J.R.: Isosteric heats of sorption of 1-naphthol and phenol from aqueous solutions by β-cyclodextrin polymers. J. Colloid Interface Sci. 307, 64–70 (2007)
Connors, K.A.: Binding Constants: the measurement of molecular complex stability, Chapter 8. Wiley, USA (1987)
Loftsson, T., Hreinsdóttir, D., Másson, M.: The complexation efficiency. J. Incl. Phenom. Macrocycl. Chem. 57, 545–552 (2007)
Gao, Y., Zhao, X., Dong, B., Zheng, L., Li, N., Zhang, S.: Inclusion complexes of β-cyclodextrin with ionic liquid surfactants. J. Phys. Chem. B 110, 8576–8581 (2006)
García-Zubiri, I.X., González-Gaitano, G., Isasi, J.R.: Sorption models in cyclodextrin polymers: Langmuir, Freundlich and a dual-mode approach. J. Colloid Interface Sci. 337, 11–18 (2009)
Acknowledgment
The authors thank the Ministerio de Ciencia e Innovación (MAT2007-65752) for financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Maddens, T., Vélaz, I., Machín, R. et al. Complexation of ebastine with β-cyclodextrin derivatives. J Incl Phenom Macrocycl Chem 70, 415–419 (2011). https://doi.org/10.1007/s10847-010-9910-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10847-010-9910-5