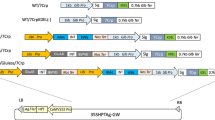
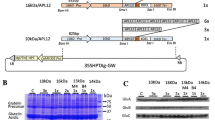
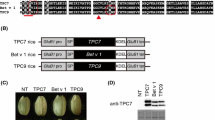
Abstract
A 7Crp peptide composed of seven major human T cell epitopes derived from the Japanese cedar pollen allergens Cry j 1 and Cry j 2 is an ideal tolerogen for peptide immunotherapy against Japanese cedar pollinosis. To maximize the accumulation level of the 7Crp peptide in transgenic rice seed, we tested endosperm specific promoters and intracellular localizations suitable for stable accumulation. A 7Crp peptide carrying the KDEL ER retention signal directed by the 2.3-kb promoter of the glutelin GluB-1, which contains a signal peptide, accumulated at the highest level of about 60 μg/grain. Notably, the 7Crp peptide predominantly accumulated in ER-derived protein bodies irrespective of the presence of various sorting signals or expression as a fusion protein with glutelin. We attribute this abnormal pattern of accumulation to the formation of disulfide bonds between the 7Crp peptide and cysteine-rich (Cys-rich) prolamin storage proteins. Furthermore, the formation of these aggregates induced the chaperone proteins BiP and PDI as an ER stress response.






Similar content being viewed by others
Abbreviations
- AGPase:
-
ADP-glucose pyrophosphorylase
- GluB-1:
-
Rice glutelin B1
- GluB-4:
-
Rice glutelin B4
- GluA-2:
-
Rice glutelin A2
- Tg:
-
Transgenic
- BiP:
-
Binding protein
- PDI:
-
Protein disulfide isomerase
- PSV:
-
Protein storage vacuole
- PB-I/II:
-
Protein body type-I/II
References
Abranches R, Arcalis E, Marcel S, Altmann F, Ribeiro-Pedro M, Rodriguez J, Stoger E (2008) Functional specialization of Medicago truncatula leaves and seeds does not affect the subcellular localization of a recombinant protein. Planta 227:649–658
Arcalis E, Marcel S, Altmann F, Kolarich D, Drakakaki G, Fischer R, Christou P, Stoger E (2004) Unexpected deposition patterns of recombinant proteins in post-endospermic reticulum compartments of wheat endosperm. Plant Physiol 136:3457–3466
Benchabane M, Goulet C, Rivard D, Faye L, Gomord V, Michaud D (2008) Preventing unintended proteolysis in plant protein biofactories. Plant Biotechnol J 6:633–648
Conrad U, Fiedler U (1998) Compartment-specific accumulation of recombinant immunoglobulins in plant cells: an essential tool for antibody production and immunomodulation of physiological functions and pathogen activity. Plant Mol Biol 38:101–109
Daniell H, Streatfield SJ, Wycoff K (2001) Medical molecular farming:production of antibodies, biopharmaceuticals and edible vaccines in plants. Trends Plant Sci 6:219–226
Drakakaki G, Marcel S, Arcalis E, Gonzalez-Melendi P, Fischer R, Christou P, Stoger E (2006) The intracellular fate of a recombinant protein is tissue dependent. Plant Physiol 141:578–586
Furtado A, Henry RJ, Takaiwa F (2008) Comparison of promoters in transgenic rice. Plant Biotechnol J 6:679–693
Goto F, Yoshihara T, Shigemoto N, Toki S, Takaiwa F (1999) Iron fortification of rice seed by the soybean ferritin gene. Nat Biotechnol 17:282–286
Gutierrez RA, MacIntosh GC, Green P (1999) Current perspectives on mRNA stability in plants: multiple levels and mechanisms of control. Trends Plant Sci 4:429–438
Hirahara K, Tatsuta T, Takatori T, Otsuka M, Kirinaka H, Kawaguchi J, Serizawa N, Taniguchi Y, Saito S, Sakaguchi M, Inouye S, Shiraishi A (2001) Preclinical evaluation of an immunotherapeutic peptide comprising 7 T-cell determinants of Cry J 1 and Cry J 2, the major Japanese cedar pollen allergens. J Allergy Clin Immunol 108:94–100
Hood EE, Kusnadi A, Nikolov Z, Howard JA (1999) Molecular farming of industrial proteins from transgenic maize. Adv Exp Med Biol 464:127–147
Hood EE, Bailey MR, Beifuss K, Magallanes-Lundback M, Horn ME, Callaway E, Drees C, Delaney DE, Clough R, Howard JA (2003) Criteria for high-level expression of fungal laccase gene in transgenic maize. Plant Biotechnol J 1:129–140
Hood EE, Love R, Lane J, Clough R, Pappu K, Drees C, Hood KR, Yoon S, Ahmad A, Howard JA (2007) Subcellular targeting is a key condition for high-level accumulation of cellulose protein in transgenic maize seed. Plant Biotechnol J 5:709–719
Kawagoe Y, Suzuki K, Tasaki M, Yasuda H, Akagi K, Katoh E, Nishizawa NK, Ogawa M, Takaiwa F (2005) The critical role of disulfide bond formation in protein sorting in the endosperm of rice. Plant Cell 17:1141–1153
Krishnan HB, Franceschi VR, Okita TW (1986) Immunochemical studies on the role of the Golgi complex in protein-body formation in rice seeds. Planta 169:471–480
Ma JK-C, Drake PMW, Christou P (2003) The production of recombinant pharmaceutical proteins in plants. Nat Rev Genet 4:794–805
Ma JK-C, Chikwamba R, Sparrow P, Fischer R, Mahoney R, Twymen RM (2005) Plant-derived pharmaceuticals-the road forward. Trends Plant Sci 10:580–585
Mainieri D, Rossi M, Architi M, Bellucci M, De Marchis F, Vavassori S, Pompa A, Arcioni S, Vitale A (2004) Zeolin. A new recombinant storage protein constructed using maize γ-zein and bean phaseolin. Plant Physiol 136:3447–3456
Nochi T, Takagi H, Yuki Y, Yang L, Masumura T, Mejima M, Nakanishi U, Matsumura A, Uozumi A, Hiroi T, Morita S, Tanaka K, Takaiwa F, Kiyono H (2007) Rice-based mucosal vaccine as a global strategy for cold-chain- and needle-free vaccination. Proc Natl Acad Sci USA 104:10986–10991
Nuttall J, Vine N, Hadlington JL, Drake P, Frigerio L, Ma JK-C (2002) ER-resident chaperon interactions with recombinant antibodies in transgenic plants. Eur J Biochem 269:6042–6051
Petruccelli S, Otegui MS, Lareu F, Tran Dinh O, Fitchette AC, Circosta A, Rumbo M, Bardor M, Carcamo R, Gomord V, Beachy RN (2006) A KDEL-tagged monoclonal antibody is efficiently retained and sorted to protein storage vacuoles in seeds. Plant Biotechnol J 4:511–527
Pompa A, Vitale A (2006) Retention of a bean phaseolin/maize r-zein fusion in the endoplasmic reticulum depends on disulfide bond formation. Plant Cell 18:2608–2621
Qu LQ, Takaiwa F (2004) Evaluation of tissue specificity and expression strength of rice seed component gene promoters in transgenic rice. Plant Biotechnol J 2:113–125
Richter LJ, Thanavala Y, Arntzen CJ, Mason HS (2000) Production of hepatitis B surface antigen in transgenic plants for oral immunization. Nat Biotechnol 18:1167–1171
Schillberg S, Twyman RM, Fischer R (2005) Opportunities for recombinant antigen and antibody expression in transgenic plants-technology assessment. Vaccine 23:1764–1769
Stoger E, Ma JK-C, Fischer R, Christou P (2005) Sowing the seeds of success: pharmaceutical proteins from plants. Curr Opin Biotechnol 16:167–173
Streatfield SJ (2007) Approaches to achieve high-level heterologous protein production in plants. Plant Biotechnol J 5:2–15
Tada Y, Utsumi S, Takaiwa F (2003) Foreign gene products can bi enhanced by introduction into low storage protein mutants. Plant Biotechnol J 1:411–422
Takagi H, Saito S, Yang L, Nagasaka S, Nishizawa N, Takaiwa F (2005a) Oral immunotherapy against a pollen allergy using seed-based peptide vaccine. Plant Biotechnol J 3:521–533
Takagi H, Hiroi T, Yang L, Tada Y, Yuki Y, Takamura K, Ishimitsu R, Kawauchi H, Kiyono H, Takaiwa F (2005b) A rice-based edible vaccine expressing multiple T cell epitopes induces oral tolerance for inhibition of Th2-mediated IgE responses. Proc Natl Acad Sci USA 102:17525–17530
Takahashi H, Saito Y, Kitagawa T, Morita S, Masumura T, Tanaka K (2005) A novel vesicle derived directly from endoplasmic reticulum is involved in the transport of vacuolar storage proteins in rice endosperm. Plant Cell Physiol 46:245–249
Takaiwa F (2007a) Transgenic rice seed as a nutriceutical delivery system. CAB reviews: perspectives in agriculture, veterinary science. Nutr Nat Resour 2:9
Takaiwa F (2007b) A rice-based edible vaccine expressing multiple T-cell epitopes to induce oral tolerance and inhibit allergy. Immunol Allergy Clin North Am 27:129–139
Takaiwa F, Ogawa M, Okita TW (1999) Rice glutelins. In: Shewry PR, Casey R (eds) Seed proteins. Kluwer Academic Publishers, Dordrecht, pp 401–425
Takaiwa F, Takagi H, Hirose S, Wakasa Y (2007) Endosperm tissue is good production platform for artificial recombinant proteins in transgenic rice. Plant Biotechnol J 5:84–92
Takaiwa F, Yang L, Yasuda H (2008a) Health-promoting transgenic rice: application of rice seeds as a direct delivery system for bioactive peptides in human health. In: Hirano HY (ed) Biotechnology in agriculture and forestry. Springer, Heidelberg, pp 357–373
Takaiwa F, Sakuta C, Choi S-K, Tada Y, Motoyama T, Utsumi S (2008b) Co-expression of soybean glycinins A1aB1b and A3B4 enhances their accumulation levels in transgenic rice seed. Plant Cell Physiol 49:1589–1599
Takemoto Y, Coughlan SJ, Okita TW, Satoh H, Ogawa M, Kumamaru T (2002) The rice mutant esp2 greatly accumulates the glutelin precursor and deletes the protein disulfide isomerase. Plant Physiol 128:1212–1222
Tanaka Y, Hayashida S, Hongo M (1975) The relationship of the feces protein particles to rice protein bodies. Agric Biol Chem 39:515–518
Tanaka K, Sugimoto T, Ogawa M, Kasai Z (1980) Isolation and characterization of two types of protein bodies in the rice endosperm. Agric Biol Chem 44:1633–1639
Torres E, Gonzalez-Melendi P, Stöger E, Shaw P, Twyman RM, Nicholson L, Vaquero C, Fischer R, Christou P, Perrin Y (2001) Native and artificial reticuloplasmins co-accumulate in distinct domains of the endoplasmic reticulum and in post-endoplasmic reticulum compartments. Plant Physiol 127:1212–1223
Twyman RM, Stoger E, Schillberg S, Christou P, Fischer R (2003) Molecular farming in plants: host systems and expression technology. Trends Biotechnol 21:570–578
Vitale A, Ceriotti A (2004) Protein quality control mechanisms and protein storage in the endoplasmic reticulum. A conflict of interests? Plant Physiol 136:3420–3426
Vitale A, Denecke J (1999) The endoplasmic reticulum—gateway of the secretory pathway. Plant Cell 11:615–628
Wakasa Y, Yasuda H, Takaiwa F (2006) High accumulation of bioactive peptide in transgenic rice seeds by expression of introduced multiple genes. Plant Biotechnol J 4:499–510
Wu CY, Adachi T, Hatano T, Washida H, Suzuki A, Takaiwa F (1998) Promoters of rice seed storage protein genes direct endosperm-specific gene expression in transgenic rice. Plant Cell Physiol 39:885–889
Yang L, Tada Y, Yamamoto MP, Zhao H, Yoshikawa M, Takaiwa F (2006) A transgenic rice seed accumulating an anti-hypertensive peptide reduces the blood pressure of spontaneously hypertensive rats. FEBS Lett 580:3315–3320
Yang L, Suzuki K, Hirose S, Wakasa Y, Takaiwa F (2007) Development of transgenic rice seed accumulating a major Japanese cedar pollen allergen (Cry j 1) structurally disrupted for oral immunotherapy. Plant Biotechnol J 5:815–826
Yang L, Kajiura H, Suzuki K, Hirose S, Fujiyama K, Takaiwa F (2008) Generation of a transgenic rice seed-based edible vaccine against house dust mite allergy. Biochem Biophys Res Comm 365:334–339
Yasuda H, Hayashi Y, Jomori T, Takaiwa F (2006) The correlation between expression and localization of a foreign gene product in rice endosperm. Plant Cell Physiol 47:756–763
Yusibov V, Rabindran S (2008) Recent progress in the development of plant-derived vaccines. Expert Rev Vaccines 7:1173–1183
Acknowledgments
We thank Dr. S. Saito (Jikei University School Medicine) for the gift of mouse monocolonal 7Crp antibody. This work was supported by the research grant, “Functional analysis of genes relevant to Agriculturally important traits in the rice genome IP2001” and “Genomics for Agricultural Innovation GMC0009” from the Ministry of Agriculture, Forestry and Fisheries of Japan to F. Takaiwa.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Takaiwa, F., Hirose, S., Takagi, H. et al. Deposition of a recombinant peptide in ER-derived protein bodies by retention with cysteine-rich prolamins in transgenic rice seed. Planta 229, 1147–1158 (2009). https://doi.org/10.1007/s00425-009-0905-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-009-0905-7