Abstract
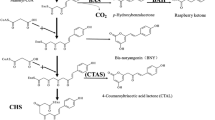
Chalcone synthase (CHS) related type III plant polyketide synthases (PKSs) are likely to be involved in the biosynthesis of diarylheptanoids (e.g. curcumin and polycyclic phenylphenalenones), but no such activity has been reported. Root cultures from Wachendorfia thyrsiflora (Haemodoraceae) are a suitable source to search for such enzymes because they synthesize large amounts of phenylphenalenones, but no other products that are known to require CHSs or related enzymes (e.g. flavonoids or stilbenes). A homology-based RT-PCR strategy led to the identification of cDNAs for a type III PKS sharing only approximately 60% identity with typical CHSs. It was named WtPKS1 (W. thyrsiflora polyketide synthase 1). The purified recombinant protein accepted a large variety of aromatic and aliphatic starter CoA esters, including phenylpropionyl- and side-chain unsaturated phenylpropanoid-CoAs. The simplest model for the initial reaction in diarylheptanoid biosynthesis predicts a phenylpropanoid-CoA as starter and a single condensation reaction to a diketide. Benzalacetones, the expected release products, were observed only with unsaturated phenylpropanoid-CoAs, and the best results were obtained with 4-coumaroyl-CoA (80% of the products). With all other substrates, WtPKS1 performed two condensation reactions and released pyrones. We propose that WtPKS1 catalyses the first step in diarylheptanoid biosynthesis and that the observed pyrones are derailment products in the absence of downstream processing proteins.









Similar content being viewed by others
Abbreviations
- BAS:
-
Benzalacetone synthase
- CHS:
-
Chalcone synthase
- IPTG:
-
Isopropyl-β-d-thiogalactopyranoside
- NAC:
-
N-acetylcysteamine
- PMSF:
-
Phenylmethanesulfonyl fluoride
- PKS:
-
Polyketide synthase
- 2PS:
-
2-Pyrone synthase
- STS:
-
Stilbene synthase
- WtPKS1:
-
Wachendorfia thyrsiflora polyketide synthase 1
References
Abe I, Sano Y, Takahashi Y, Noguchi H (2003) Site-directed mutagenesis of benzalacetone synthase. J Biol Chem 278:25218–25226
Austin MB, Noel JP (2003) The chalcone synthase superfamily of type III polyketide synthases. Nat Prod Rep 20:79–110
Austin MB, Bowman ME, Ferrer JL, Schröder J, Noel JP (2004a) An aldol switch discovered in stilbene synthases mediates cyclization specificity of type III polyketide synthases. Chem Biol 11:1179–1194
Austin MB, Izumikawa M, Bowman M, Udwary D, Ferrer J-L, Moore B, Noel J (2004b) Crystal structure of a bacterial type III polyketide synthase and enzymatic control of reactive polyketide intermediates. J Biol Chem 279:45162–45174
Baranovsky A, Schmitt B, Fowler DJ, Schneider B (2003) Synthesis of new biosynthetically important diarylheptanoids and their oxa- and fluoro analogues by three different strategies. Synth Commun 33:1019–1045
Bazan AC, Edwards JM, Weiss U (1978) Synthesis of lachnanthocarpone [9-phenyl-2,6-dihydroxyphenalen-1(6)-one] by intramolecular Diels–Alder cyclization of a 1,7-diarylheptanoid orthoquinone; possible biosynthetic significance of Diels–Alder reactions. Tetrahedron 34:3005–3015
Beuerle T, Pichersky E (2002) Enzymatic synthesis and purification of aromatic coenzyme A esters. Arch Biochem Biophys 302:305–312
Bick IRC, Blackman AJ (1973) Haemodorin—a phenalenone pigment from Haemodorum distichophyllum. Aust J Chem 26:1377–1380
Bomati EK, Austin MB, Bowman E, Dixon RA, Noel JP (2005) Structural elucidation of chalcone reductase and implications for deoxychalcone biosynthesis. J Biol Chem 280:30496–30503
Borejsza-Wysocki W, Hrazdina G (1994) Biosynthesis of p-hydroxyphenylbutan-2-one in raspberry fruits and tissue cultures. Phytochemistry 35:623–628
Bradford MM (1976) Rapid and sensitive method for quantitation of microgram quantities of protein utilizing principle of protein-dye binding. Anal Biochem 72:248–254
Chattopadhyay I, Biswas K, Bandyopadhyay U, Banerjee RK (2004) Turmeric and curcumin: biological actions and medicinal applications. Curr Sci India 87:44–53
Dora GA (1991) Phytochemical study of some Haemodoraceous plants. PhD thesis, University of Connecticut
Eckermann S, Schröder G, Schmidt J, Strack D, Edrada RA, Helariutta Y, Elomaa P, Kotilainen M, Kilpelainen I, Proksch P, Teeri TH, Schröder J (1998) New pathway to polyketides in plants. Nature 396:387–390
Eckermann C, Schröder G, Eckermann S, Strack D, Schmidt J, Schneider B, Schröder J (2003) Stilbenecarboxylate biosynthesis: a new function in the family of chalcone synthase-related proteins. Phytochemistry 62:271–286
Edwards JM (1974) Phenylphenalenones from Wachendorfia species. Phytochemistry 13:90–291
Ferrer JL, Jez JM, Bowman ME, Dixon RA, Noel JP (1999) Structure of chalcone synthase and the molecular basis of plant polyketide biosynthesis. Nat Struct Biol 6:775–784
Funa N, Ohnishi Y, Ebizuka Y, Horinouchi S (2002) Properties and substrate specificity of RppA, a chalcone synthase-related polyketide synthase in Streptomyces griseus. J Biol Chem 277:4628–4635
Gilbert IH, Ginty M, O’Neill JA, Simpson TJ, Staunton J, Willis CL (1995) Synthesis of β-keto and α,β-unsaturated N-acetylcysteamine thioesters. Bioorg Med Chem Lett 5:1587–1590
Helariutta Y, Elomaa P, Kotilainen M, Griesbach RJ, Schröder J, Teeri TH (1995) Chalcone synthase-like genes active during corolla development are differentially expressed and encode enzymes with different catalytic properties in Gerbera hybrida (Asteraceae). Plant Mol Biol 28:47–60
Hölscher D (1996) Biosynthese und Strukturermittlung von Phenylphenalenonen aus Anigozanthos preissii Endl. PhD thesis, Martin Luther University Halle
Hölscher D, Schneider B (1995) A diarylheptanoid intermediate in the biosynthesis of phenylphenalenones in Anigozanthos preissii. J Chem Soc Chem Commun 525–526
Hölscher D, Schneider B (1996) A resveratrol dimer from Anigozanthos preissii and Musa Cavendish. Phytochemistry 43:471–473
Hölscher D, Schneider B (1997) Phenylphenalenones from root cultures of Anigozanthos preissii. Phytochemistry 45:87–91
Jez JM, Austin MB, Ferrer J, Bowman ME, Schröder J, Noel JP (2000a) Structural control of polyketide formation in plant-specific polyketide synthases. Chem Biol 7:919–930
Jez JM, Ferrer J-L, Bowman ME, Dixon RA, Noel JP (2000b) Dissection of malonyl-coenzyme A decarboxylation from polyketide formation in the reaction mechanism of a plant polyketide synthase. Biochemistry 39:890–902
Jez JM, Bowman ME, Noel JP (2002) Expanding the biosynthetic repertoire of plant type III polyketide synthases by altering starter molecule specificity. Proc Natl Acad Sci USA 99:5319–5324
Junghans H, Dalkin K, Dixon RA (1993) Stress responses in alfalfa (Medicago sativa L.). 15. Characterization and expression patterns of members of a subset of the chalcone synthase multigene family. Plant Mol Biol 22:239–253
Kamo T, Hirai N, Tsuda M, Fujioka D, Ohigashi H (2000) Changes in the content and biosynthesis of phytoalexins in banana fruit. Biosci Biotech Biochem 64:2089–2098
Lanz T, Tropf S, Marner F-J, Schröder J, Schröder G (1991) The role of cysteines in polyketide synthases: site-directed mutagenesis of resveratrol and chalcone synthases, two key enzymes in different plant-specific pathways. J Biol Chem. 266:9971–9976
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Opitz S (2002) Phenylphenalenones and related phenolic pigments of the Haemodoraceae: structure, biosynthesis and accumulation patterns in Xiphidium caeruleum and Wachendorfia thyrsiflora. PhD thesis, Friedrich Schiller University Jena
Opitz S, Schneider B (2003) Oxidative biosynthesis of phenylbenzoisochromenones from phenylphenalenones. Phytochemistry 62:307–312
Opitz S, Otálvaro F, Echeverri F, Quiñones W, Schneider B (2002) Isomeric oxabenzochrysenones from Musa acuminata and Wachendorfia thyrsiflora. Nat Prod Lett 16:335–338
Saitou N, Nei M (1987) The neighbour-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Schmitt B, Schneider B (1999) Dihydrocinnamic acids are involved in the biosynthesis of phenylphenalenones in Anigozanthos preissii. Phytochemistry 52:45–53
Schröder J (1997) A family of plant-specific polyketide synthases: facts and predictions. Trends Plant Sci 2:373–378
Schröder J (2000) The family of chalcone synthase-related proteins: functional diversity and evolution. Recent Adv Phytochem 34:55–89
Schröder J, Raiber S, Berger T, Schmidt A, Schmidt J, Soares-Sello AM, Bardshiri E, Strack D, Simpson TJ, Veit M, Schröder G (1998) Plant polyketide synthases: a chalcone synthase-type enzyme which performs a condensation reaction with methylmalonyl-CoA in the biosynthesis of C-methylated chalcones. Biochemistry 37:8417–8425
Schüler G, Mithöfer A, Baldwin IT, Berger S, Ebel J, Santos JG, Herrmann G, Hölscher D, Kramell R, Kutchan TM, Maucher H, Schneider B, Stenzel I, Wasternack C, Boland W (2004) Coronalon: a powerful tool in plant stress physiology. FEBS Lett 563:17–22
Schwede T, Kopp J, Guex N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modeling server. Nucleic Acids Res 31:3381–3385
Thomas R (2001) A biosynthetic classification of fungal and streptomycete fused-ring aromatic polyketides. ChemBioChem 2:612–627
Van de Peer Y, De Wachter R (1994) TREECON for Windows: a software package for the construction and drawing of evolutionary trees for the Microsoft Windows environment. Comput Appl Biosci 10:569–570
Wang CZ, Maier UH, Zenk MH (2000) Synthesis of 3,3′,5-trihydroxybiphenyl-2-carboxylic acid, a component of the bitterest natural product amarogentin and its coenzyme A and N-acetyl cysteamine thiol esters. J Nat Prod 63:371–374
Welle R, Schröder G, Schiltz E, Grisebach H, Schröder J (1991) Induced plant responses to pathogen attack: analysis and heterologous expression of the key enzyme in the biosynthesis of phytoalexins in soybean (Glycine max L. Merr. cv. Harosoy 63). Eur J Biochem 196:423–430
Zhu JP, Islas-Gonzales G, Bois-Choussy M (2000) Recent progress in isolation, bioactivity evaluation and total synthesis of diarylheptanoids. Org Prep Proced Int 32:505–546
Acknowledgements
The authors wish to thank Gudrun Schröder (Freiburg) for protein expression and practical advice and J.P. Noel (La Jolla) for providing the pHis8 vector. We are grateful to A. Schmidt and M. Reichelt (Jena) for assistance in radio-HPLC analysis and Bettina Raguschke (Jena) for DNA sequencing. This work was financially supported by the Deutsche Forschungsgemeinschaft (Schn 450–4).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Brand, S., Hölscher, D., Schierhorn, A. et al. A type III polyketide synthase from Wachendorfia thyrsiflora and its role in diarylheptanoid and phenylphenalenone biosynthesis. Planta 224, 413–428 (2006). https://doi.org/10.1007/s00425-006-0228-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-006-0228-x