Abstract
Objectives
Poor bone quality increases the susceptibility to fractures of the proximal humerus. It is unclear whether local trabecular and cortical measures influence the severity of fracture patterns. The goal of this study was to assess parameters of trabecular and cortical bone properties and to compare these parameters with the severity of fractures and biomechanical testing.
Methods
Twenty patients with displaced proximal humeral fractures planned for osteosynthesis were included. Fractures were classified as either 2-part fractures or complex fractures. Bone after core drilling was harvested during surgery from the humeral head in each patient. Twenty bone cores obtained from nonpaired cadaver humeral heads served as nonfractured controls. Micro-CT (μCT) was performed and bone volume/total volume (BV/TV), connectivity density (CD), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and bone mineral density (BMD) were assessed. The cortical index (CI) was determined from AP plain films. Biomechanical testing was done after μCT scanning by axially loading until failure, and ultimate strength and E modulus were recorded.
Results
BV/TV, BMD and CD showed moderate to strong correlations with biomechanical testing (r = 0.45–0.76, all p < 0.05). No significant differences were detected between the 2-part and complex fracture groups and controls regarding μCT and biomechanical parameters. CI was not significantly different between the 2-part and complex fracture groups.
Conclusions
In our study population local trabecular bone structure and cortical index could not predict the severity of proximal humeral fractures in the elderly. Complex fractures do not necessarily imply lower bone quality compared to simple fractures.
Similar content being viewed by others
Introduction
Fractures of the proximal humerus are considered osteoporotic fractures. Especially geriatric women are affected by fractures of the proximal humerus [1], which is attributed to the high prevalence of low-bone quality and the elevated risk of falling in this age group [2]. The mechanical stability of bone is mainly influenced by the local amount and composition of cortical and trabecular bone. It has been suggested that patients with osteoporotic proximal humeral fractures have more complex fractures, but this conclusion was based only on descriptive data and not on quantitative measurements [3].
Different standard techniques such as dual-energy X-ray absorptiometry (DXA) or quantitative computed tomography (QCT) exist to assess bone mineral density (BMD) as a measure for the quantity of bone. A 10% loss of bone mass doubles the risk of a vertebral fracture or leads to a 2.5 times higher risk of a hip fracture [4]. Even though BMD is a widely accepted parameter for assessing bone stability, other factors not captured by densitometry contribute to bone quality as well. The structure and micro-architecture of bone contribute significantly to its mechanical competence [5]. It has been proposed that a low amount of trabecular bone leads to reduced stability. Assessment of structural properties can improve the prediction of bone strength and fracture risk [6–9] and may also help to explain different fracture patterns. However, investigating the influence of local bone parameters on the complexity of fractures is challenging, because in vivo assessment is technically limited by the available techniques. In vivo multi-detector computed tomography techniques can assess parameters derived from bone architecture, but standardized methods are still not available for clinical use [5, 10]. Micro-CT (μCT) has become an in vitro standard technique for the measurement of structural parameters, but it requires bone biopsy, which is difficult to justify and perform in vivo. This may contribute to the fact that the influence of bone structure on the complexity of fracture patterns in the proximal humerus has not been investigated yet.
The goal of this in vivo study was to use μCT and radiographs to assess local trabecular structure and cortical thickness in patients with proximal humeral fractures to then compare these parameters with the severity of fractures and with nonfractured controls.
Materials and methods
Patients and specimen
Twenty patients planned for intramedullary nailing or humeral head replacement for displaced proximal humeral fracture were included in this study (5 males, 15 females). The mean age was 73 years (range 52–96 years). The study was approved by the institutional ethics committee (affiliation 1).
Prior to the surgery, standard axial and AP radiographs were taken. The type of fracture was then classified as “2-part” or “complex” by the senior author. According to the type of fracture, seven patients were assigned to the 2-part group (mean age of 70 years, range 52–91 years; 6 women, 1 man); 13 patients presented with complex fractures of the humeral head (mean age of 75 years, range 52–96 years; 9 women, 4 men). Six/seven patients in the 2-part group and 7/13 in the complex group were treated by intramedullary nailing. In 1/7 patients in the 2-part group and 6/13 in the complex group, humeral head replacement was performed. The average time between trauma and surgery was 3.9 days in the 2-part group and 3.4 days in the complex group.
A direct low-height fall on the shoulder was the most frequent trauma in both groups (2-part: 6/7, complex: 7/13). In the complex group, though, 4 patients presented after a stair fall, while 1 patient fell on her extended arm, and in one case trauma mechanism was not identifiable. None of the patients was involved in a high-speed trauma (i.e. car accident).
Twenty body donors were included in the study as controls (11 females, 9 males). The mean age was 82 years (range 56–94 years). All individuals had given written consent to donate their bodies to the Institute of Anatomy after death, in accordance with local legal requirements.
Patients and donors with radiographic or macroscopic signs of previous ipsilateral fractures of the humerus or an osseous tumor were excluded.
Assessment of bone properties
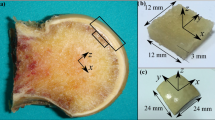
In patients planned for intramedullary nailing, bony drilling cores of 8 mm diameter (Fig. 1b) were obtained while trepanning the entrance for the nail (Targon PH, 10/220 mm, Aesculap, Tuttlingen, Germany) [11]. Similarly, drilling cores were harvested from humeral heads removed from patients undergoing endoprosthetic head replacement. These bone specimens accrue under normal circumstances during these operations. By this, no additional damage done by taking the samples. Analogously, 20 drilling cores were harvested from nonpaired cadaver humeral heads. Care was taken that all cores were obtained in the same location at the apex of the humeral head (the usual nail entry point). Subsequently, the samples were fixed in ethanol 70% for at least 3 weeks.
Parameters of bone structure were measured using a μCT imaging system (μCT40, Scanco Medical, Bassersdorf, Switzerland) that was equipped with a 5 μm focal spot X-ray tube as a source. The X-ray tube was operated at 70 kV and 114 μA, and integration time was set to 200 ms. Two-dimensional CT images were reconstructed in 1,024 × 1,024 pixel matrices from 500 projections using a standard convolution-backprojection procedure. Images were stored in 3D arrays with an isotropic voxel size of 20 μm. The long axis of the biopsies from the proximal humerus was orientated orthogonal to the axis of the X-ray beam. Depending on the original length of the biopsy and starting at the most distal end of the subchondral bone, 250 to 400 micro-tomographic slices were acquired, corresponding to a total length of 5–8 mm. During the scanning procedure biopsies were immersed in ethanol 70%.
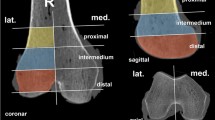
Bone density evaluation and morphometric analysis were carried out for a cylindrical region of interest (ROI) with a thickness of 4 mm and a diameter of 8 mm using image processing software provided by the scanner manufacturer (IPL, Scanco Medical, Bassersdorf, Switzerland). The ROI was set 1 mm below the most distal cartilage border to exclude the proximal subchondral bone (Fig. 1a). A Gaussian filter with a sigma of 0.7 and a support of one voxel was first used to suppress noise. To obtain binarized images the same segmentation threshold for all samples was selected at 24% of the maximal gray scale value, which corresponded to the peak for bone tissue in the histogram of the gray value distribution (Figs. 2, 3). For the resulting volumes containing exclusively cancellous bone, the following parameters were assessed: bone volume/total volume (BV/TV), connectivity density (CD), trabecular number (Tb.N), trabecular thickness (Tb.Th), trabecular spacing (Tb.Sp), and bone mineral density (BMD)
Examples of five gray level histograms with the threshold chosen for all samples. Note the threshold of 240 separating clearly bone and background noise. The histograms of these five samples are representative for all examinations. For 3D reconstructions of “H. W.”, “M. N.” and “N. U., female, 81y” see Fig. 4
In addition to these structural parameters of trabecular bone, the cortical index (CI) was measured from AP radiographs in the 2-part and complex fracture groups using a technique described previously [12].
Biomechanical testing
After μCT scanning, biomechanical testing was performed with a material testing machine (5500, Instron, Bucks, UK). According to the ROI analyzed by μCT, a cylinder of 4 mm length was cut 1 mm below the most distal cartilage border. The diameter was left at 8 mm. The samples were loaded axially to failure (i.e. loss of resistance), and ultimate load (UL; maximum load before failure) and elasticity modulus (EM; stress divided by strain in the elastic region) were recorded. For all calculations, testing software provided by the machine’s manufacturer was used (Bluehill 2, Instron, Bucks, UK).
Statistical analysis
Descriptive data are expressed as mean ± standard deviation. A one-way ANOVA with a Bonferroni post hoc was used to detect differences between the three groups. As the mean age differed between the three groups, an ANCOVA was used to exclude age as confounding factor. Correlations were calculated using Pearson’s correlation coefficient r. For correlations with age, the two fracture groups were pooled. Correlation was considered weak for ∣r∣ < 0.5, moderate for ∣r∣ < 0.7, strong for ∣r∣ < 0.9, and very strong for ∣r∣ > 0.9 [13]. All differences were considered significant for values of p < 0.05. Statistical analysis of all data was performed with SPSS 14.0 (Chicago, IL, USA).
Results
μCT imaging—trabecular structure
The μCT imaging analysis showed no significant differences between the three groups for any of the calculated parameters (one-way ANOVA: BV/TV: p = 0.83; CD: p = 0.78; Tb.N: p = 0.59; Tb.Th: p = 0.44; Tb.Sp: p = 0.29; BMD: p = 0.75) (Table 1). There was a moderate correlation between age and BV/TV (r = 0.65, p = 0.002) and age and BMD (r = 0.65, p = 0.002) in the control group, but this was not seen in either of the fracture groups. Except for Tb.N versus Tb.Th (r = 0.02) and CD versus Tb.Th (r = 0.19), there was a moderate to very strong correlation between the single μCT parameters (r range ± [0.52–0.98]).
Cortical index
The 2-part group had a mean CI of 0.29 ± 0.09, and the complex group had an average CI of 0.28 ± 0.08. There was no significant difference between the two groups (p = 0.85). Except for one patient, all measured indices were below 0.40.
Biomechanical testing
Comparable to the μCT evaluation, no significant differences between the groups 2-part, complex, and controls were detected for the biomechanical parameters UL and EM (one-way ANOVA; UL: p = 0.32; EM: p = 0.16) (Table 2). However, there was a moderate to strong correlation between the biomechanical parameters and some of the μCT parameters: UL showed a strong correlation to BV/TV (r = 0.76, p < 0.001) and a moderate correlation to BMD (r = 0.69, p < 0.001) and CD (r = 0.52, p < 0.001); correlations between UL and Tb.N, Tb.Th, and Tb.Sp were only weak (r = 0.40, 0.37, −0.39; all p < 0.05). The EM correlated moderately with BV/TV (r = 0.52, p = 0.001) and BMD (r = 0.45, p = 0.004).
While there was no correlation between age and any of the biomechanical parameters in either the 2-part or the complex fracture group, age showed a moderate correlation with ultimate load (r = 0.63, p = 0.003) and E modulus (r = 0.50, p = 0.023) in the control group.
When excluding age as covariate by ANCOVA, again no significant differences were observed between the groups for any of the parameters mentioned above.
Discussion
The present study is the first study investigating the trabecular bone structure of bone samples obtained in vivo from patients with proximal humeral fractures. Contrary to the authors’ hypothesis, no significant difference was detected between 2-part and complex fractures and nonfractured controls, neither for the μCT nor for the biomechanical parameters. More complex fractures do not seem to imply a lower quality of cancellous bone or inferior load capacities. Thus, these results indicate that the severity of a proximal humeral fracture depends on other factors than the local trabecular structure or cortical index.
Fracture types were classified by one observer in accordance with standard clinical practice. The readings were done by the senior author of this study, who has 15 years of experience in trauma and orthopedic surgery. We chose to differentiate only between complex and 2-part fractures, avoiding the constraints of classifications such as AO or Neer [14].
Bone samples were extracted during surgery in a standardized manner. However, obtaining bone samples in living patients cannot be as accurate as in body donors due to intraoperative constraints. This may lead to some variation in the site of bone biopsy and its structural properties [15, 16]. For ethical reasons, it was not possible to obtain multiple bone cores from different sites, as this may have impaired the stability of the implants following resection of the bone core. It is known that the sampling site used in our study approximately represents the average BMD and indentation stiffness of the whole trabecular proximal humerus [15]. However, it has to be considered that there are different changes in different sites of the humerus with age [17].
All bone samples were fixed in ethanol 70% for at least 3 weeks to equalize possible alterations by storage duration. In contrast to formaldehyde, storage in ethanol does not change the stiffness of trabecular bone [18]. Longer storage may have influenced the radio-opacity of the inter-trabecular fat, even though it can be assumed that the chosen threshold still separated bone from background noise sufficiently (Fig. 4).
Examples of 3D reconstructions of the scanned ROI. a H. W., female, 58 years, simple fall on the shoulder, “2part”: BV/TV 0.09, CI 0.16. b M. N., female, 56 years, stair fall, “complex”: BV/TV 0.20, CI 0.30. c N. U., female, 81 years, no trauma, “control”: BV/TV 0.14. Note the lower bone quality of the “2part”-sample when compared to the “complex”-sample
Bone mineral density measured using standard bone densitometers is limited in predicting the fracture risk and bone quality. One-third of bone strength remains unexplained when only density is considered [19]. Parameters of bone structure provide valuable additional information for assessing bone quality [5, 20]. We used an isotropic voxel size of 20 μm. Even though comparable resolutions have been used before [10, 21, 22], smaller voxel sizes would allow a better interpretation of especially the morphometric parameters (Tb.N, Tb.Th, CD).
It has been shown that trabecular bone parameters obtained by μCT can predict biomechanical strength [23]. Trabecular number and also trabecular thickness seem to contribute significantly to bone strength. A loss of bone with aging primarily affects the overall number of trabeculae and the connectivity [24].
This implies a limitation of our study as the three groups differed in mean age and it is known that BV/TV changes with age [15]. Yet, even when age was excluded as influencing factor, no significant differences were seen between the groups. Actually, older patients with complex fractures had rather dense and biomechanical stiff bone.
Our results on trabecular bone structure of the proximal humerus are comparable to the data reported in different in vitro and in vivo studies of other body regions [25–29]. Subchondral bone harvested in hip joints with osteoarthritis showed more than about twice the BV/TV and Tb.N of our bone cores [26, 28]. However, these data refer to the trabecular bone of the femoral head, where much higher forces and stresses are present. Nonweight-bearing bone like the iliac crest, in contrast, seems to have quite similar BV/TV, Tb.N and Tb.Th when compared to the proximal humerus [27].
In accordance with previously published data patients with more complex humeral fractures were older [3]. However, comparison of the data obtained in patients with simple and complex fractures with those from the nonfractured controls indicates that trabecular bone structure can explain neither the occurrence nor the severity of a proximal humeral fracture (Fig. 4). Patients who sustain proximal humeral fractures have higher levels of physical fitness than those with proximal femoral fractures [3] but are less fit than patients presenting with distal radial fractures [30]. This indicates that trauma mechanism and neuromuscular coordination appear to strongly influence the sites at which fractures occur [31]. In our study, the mechanisms and intensities of the trauma which led to the fractures were considerably different between the 2-part and complex group. Almost all patients in the 2-part group had only a simple fall, whereas half of the patients in the complex group were involved in falls from a certain height, which in some cases also led to concomitant fractures. Thus, our data suggest that the trauma mechanism may have more influence on fracture severity than the local bone structure. The forces acting during several fracture mechanisms are to be further investigated in terms of direction and intensity. In addition, it is known and consistent with our results that proximal humeral fractures are associated with a CI below 0.4 [12]. The cortical bone strength may be of influence on fracture morphology and it should also be kept in mind that neither cortical nor cancellous bone is a separate structure. More complex models may be necessary to interpret the influence of bone morphology on stability [32].
In conclusion, in our study population local trabecular bone structure and cortical index could not predict the severity of proximal humeral fractures in the elderly.
Our results suggest that other factors than local bone properties seem to have a great impact on the development of more complex fractures. Further studies with larger numbers of patients and bone samples from different sites within the humeral head are required to confirm our data.
References
Maravic M, Le Bihan C, Landais P, Fardellone P (2005) Incidence and cost of osteoporotic fractures in France during 2001. A methodological approach by the national hospital database. Osteoporos Int 16:1475–1480
Lee S, Dargent-Molina P, Bréart G (2002) EPIDOS Group. Epidemiologie de l’Osteoporose Study. Risk factors for fractures of the proximal humerus: results from the EPIDOS prospective study. J Bone Miner Res 17:817–825
Court-Brown C, Garg A, McQueen M (2001) The epidemiology of proximal humeral fractures. Acta Orthop Scand 72:365–371
Klotzbuecher C, Ross P, Landsman P, Abbott T, Berger M (2000) Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res 15:721–739
Diederichs G, Link TM, Kentenich M, Schwieger K, Huber MB, Burghardt AJ, Majumdar S, Rogalla P, Issever AS (2009) Assessment of trabecular bone structure of the calcaneus using multi-detector CT: correlation with microCT and biomechanical testing. Bone 44:976–983
Wigderowitz CA, Paterson CR, Dashti H, McGurty D, Rowley DI (2000) Prediction of bone strength from cancellous structure of the distal radius: can we improve on DXA? Osteoporos Int 11:840–846
Link TM, Majumdar S, Lin JC, Newitt D, Augat P, Ouyang X, Mathur A, Genant HK (1998) A comparative study of trabecular bone properties in the spine and femur using high resolution MRI and CT. J Bone Miner Res 13:122–132
Link TM, Majumdar S, Lin JC, Augat P, Gould RG, Newitt D, Ouyang X, Lang TF, Mathur A, Genant HK (1998) Assessment of trabecular structure using high resolution CT images and texture analysis. J Comput Assist Tomogr 22:15–24
Ito M, Ohki M, Hayashi K, Yamada M, Uetani M, Nakamura T (1997) Relationship of spinal fracture to bone density, textural, and anthropometric parameters. Calcif Tissue Int 60:240–244
Issever AS, Link TM, Kentenich M, Rogalla P, Schwieger K, Huber MB, Burghardt AJ, Majumdar S, Diederichs G (2009) Trabecular bone structure analysis in the osteoporotic spine using a clinical in vivo setup for 64-slice MDCT imaging: comparison to microCT imaging and microFE modeling. J Bone Miner Res 24:1628–1637
Mittlmeier TW, Stedtfeld HW, Ewert A, Beck M, Frosch B, Gradl G (2003) Stabilization of proximal humeral fractures with an angular and sliding stable antegrade locking nail (Targon PH). J Bone Jt Surg Am 85-A:136–146
Hepp P, Theopold J, Osterhoff G, Marquass B, Voigt C, Josten C (2009) Bone quality measured by the radiogrammetric parameter “cortical index” and reoperations after locking plate osteosynthesis in patients sustaining proximal humerus fractures. Arch Orthop Trauma Surg 129:1251–1259
Bühl A, Zöfel P (2002) SPSS 11: Einführung in die moderne Datenanalyse unter Windows. Pearson Studium, Munich
Siebenrock K, Gerber C (1993) The reproducibility of classification of fractures of the proximal end of the humerus. J Bone Jt Surg 75:1751–1755
Lill H, Hepp P, Gowin W, Oestmann J, Korner J, Haas N, Josten C, Duda G (2002) Age- and gender-related distribution of bone mineral density and mechanical properties of the proximal humerus. Rofo 174:1544–1550
Hepp P, Lill H, Bail H, Korner J, Niederhagen M, Haas NP, Josten C, Duda GN (2003) Where should implants be anchored in the humeral head? Clin Orthop Relat Res 415:139–147
Barvencik F, Gebauer M, Beil FT, Vettorazzi E, Mumme M, Rupprecht M, Pogoda P, Wegscheider K, Rueger JM, Pueschel K, Amling M (2009) Age- and sex-related changes of humeral head microarchitecture: histomorphometric analysis of 60 human specimens. J Orthop Res 28:18–26
Linde F, Sørensen HCF (1993) The effect of different storage methods on the mechanical properties of trabecular bone. J Biomech 26:1249–1252
Ammann P, Rizzoli R (2003) Bone strength and its determinants. Osteoporos Int 14(Suppl 3):S13–S18
Wachter NJ, Augat P, Mentzel M, Sarkar MR, Krischak GD, Kinzl L, Claes LE (2001) Predictive value of bone mineral density and morphology determined by peripheral quantitative computed tomography for cancellous bone strength of the proximal femur. Bone 28:133–139
Perilli E, Baruffaldi F, Visentin M, Bordini B, Traina F, Cappello A, Viceconti M (2007) MicroCT examination of human bone specimens: effects of polymethylmethacrylate embedding on structural parameters. J Microsc 225:192–200
Diederichs G, Korner J, Goldhahn J, Linke B (2006) Assessment of bone quality in the proximal humerus by measurement of the contralateral site: a cadaveric analyze. Arch Orthop Trauma Surg 126:93–100
Goulet R, Goldstein S, Ciarelli M, Kuhn J, Brown M, Feldkamp L (1994) The relationship between the structural and orthogonal compressive properties of trabecular bone. J Biomech 27:375–389
Mittra E, Rubin C, Gruber B, Qin YX (2008) Evaluation of trabecular mechanical and microstructural properties in human calcaneal bone of advanced age using mechanical testing, microCT, and DXA. J Biomech 41:368–375
Chen H, Zhou X, Shoumura S, Emura S, Bunai Y (2010) Age- and gender-dependent changes in three-dimensional microstructure of cortical and trabecular bone at the human femoral neck. Osteoporos Int 21:627–636
Zhang ZM, Li ZC, Jiang LS, Jiang SD, Dai LY (2010) Micro-CT and mechanical evaluation of subchondral trabecular bone structure between postmenopausal women with osteoarthritis and osteoporosis. Osteoporos Int 21:1383–1390
Chappard C, Marchadier A, Benhamou L (2008) Interindividual and intraspecimen variability of 3-D bone microarchitectural parameters in iliac crest biopsies imaged by conventional micro-computed tomography. J Bone Miner Metab 26:506–513
Chappard C, Peyrin F, Bonnassie A, Lemineur G, Brunet-Imbault B, Lespessailles E, Benhamou C (2006) Subchondral bone micro-architectural alterations in osteoarthritis: a synchrotron micro-computed tomography study. Osteoarthr Cartil 14:215–223
Djuric M, Djonic D, Milovanovic P, Nikolic S, Marshall R, Marinkovic J, Hahn M (2010) Region-specific sex-dependent pattern of age-related changes of proximal femoral cancellous bone and its implications on differential bone fragility. Calcif Tissue Int 86:192–201
Kelsey J, Browner W, Seeley D, Nevitt M, Cummings S (1992) Risk factors for fractures of the distal forearm and proximal humerus. Am J Epidemiol 135:477–489
Palvanen M, Kannus P, Parkkari J, Pitkajarvi T, Pasanen M, Vuori I, Jarvinen M (2000) The injury mechanisms of osteoporotic upper extremity fractures among older adults: a controlled study of 287 consecutive patients and their 108 controls. Osteoporos Int 11:822–831
Seebeck J, Goldhahn J, Städele H, Messmer P, Morlock M, Schneider E (2004) Effect of cortical thickness and cancellous bone density on the holding strength of internal fixator screws. J Orthop Res 22:1237–1242
Acknowledgments
The authors declare that the experiments described above comply with the current laws of the countries in which they were performed.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Osterhoff, G., Diederichs, G., Tami, A. et al. Influence of trabecular microstructure and cortical index on the complexity of proximal humeral fractures. Arch Orthop Trauma Surg 132, 509–515 (2012). https://doi.org/10.1007/s00402-011-1446-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00402-011-1446-7