Abstract.
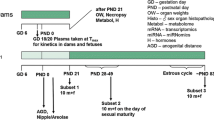
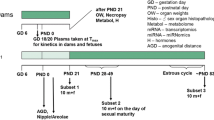
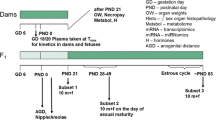
Two repeated-dose studies of 6-n-propyl-2-thiouracil (PTU) in male rats based on the research protocol 'Pubertal Development and Thyroid Function in Immature Male Rats' (pubertal assay) proposed by the Endocrine Disrupter Screening and Testing Advisory Committee (EDSTAC) and the draft protocol of the 'Enhanced OECD Test Guideline 407' (enhanced TG 407) were performed to investigate the suitability of both assays as screening methods for the detection of endocrine-mediated effects and to compare their sensitivity for the endocrine-mediated effects. In the pubertal assay, PTU at doses of 0, 0.01, or 1 mg/kg per day was orally administered to male Sprague-Dawley rats for 30 days, starting at 23 days of age. In the enhanced TG 407 the same doses of PTU were orally administered to male Sprague-Dawley rats for 28 days, starting at 7 weeks of age. In the pubertal assay, decreased serum thyroxine (T4) and triiodothyronine (T3), increased thyroid and pituitary weights, hypertrophy of follicular epithelial cells in the thyroid, and increased basophilic cells in the pituitary were detected as endocrine-mediated effects of PTU in the 1 mg/kg group. In the enhanced TG 407, decreased T4 and T3 were detected in both the 0.01 and 1 mg/kg groups, together with increased thyroid-stimulating hormone in the 1 mg/kg group, increased thyroid and pituitary weights in the 1 mg/kg group, and hypertrophy of follicular epithelial cells in the thyroid and increased basophilic cells in the pituitary of the 1 mg/kg group. Thus, among the parameters tested, the thyroid hormone levels, organ weight changes, and the histopathological assessment allowed detection of the endocrine-related effects of PTU in both the pubertal assay and the enhanced TG 407, but the sensitivity of the hormone analysis was higher in the latter.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Electronic Publication
Rights and permissions
About this article
Cite this article
Yamasaki, K., Tago, Y., Nagai, K. et al. Comparison of toxicity studies based on the draft protocol for the 'Enhanced OECD Test Guideline no. 407' and the research protocol of 'Pubertal Development and Thyroid Function in Immature Male Rats' with 6-n-propyl-2-thiouracil. Arch Toxicol 76, 495–501 (2002). https://doi.org/10.1007/s00204-002-0371-5
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s00204-002-0371-5