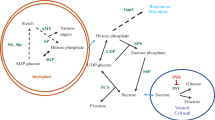
Abstract
The benefits of being able to process potatoes directly into chips or fries from cold storage (2 to 4 C) include less shrinkage, retention of dry matter, decreased disease loss, extended marketability, and the elimination of the need for dormancy-prolonging chemicals. Unfortunately at low temperature, potato tubers undergo a phenomenon known as cold-induced sweetening where the rate of conversion of starch to reducing sugars (i.e., glucose and fructose) is accelerated. As raw potatoes are sliced and cooked in oil at high temperature, the accumulated reducing sugars react with free amino acids in the potato cell forming unacceptably brown- to black-pigmented chips or fries via a non-enzymatic, Maillard-type reaction. Potatoes yielding these unacceptably colored products are generally rejected for purchase by the processing plant. All commercial potato cultivars presently used for the production of potato chips and fries accumulate excess free reducing sugars when exposed to cold stress. If a “cold-processing potato” was available, energy savings would be realized in potato-growing regions where outside storage temperatures are cool. In regions where outside temperatures are moderately high, increased refrigeration costs may occur. This expense would be offset, however, by removal of the need to purchase dormancy-prolonging chemicals, by a decreased need for disease control and by improvement of long-term tuber quality. The primary goal of this review is to describe recent research of a biochemical and molecular nature that relates to the underlaying mechanisms regulating post harvest, cold-induced sweetening in potato tubers. No attempt was made to outline the extensive research conducted on the genetic manipulation of carbon metabolism between starch and free sugars during photosynthesis and/or during potato development in relation to source/sink interactions.
Resumen
Los beneficios que se obtienen al procesar papas fritas o en houjuelas de manera directa, que hayan estado almacenadas en cámaras frigoríficas a temperaturas que van de 2 a 4°C, incluyen menor encogimiento, retención de sustancia seca, disminución de enfermedades, un amplio potencial para el mercado y la eliminación de la necesidad de prolongar el estado de dormancia mediante químicos. Desgraciadamente, a bajas temperatoras, los tubérculos de la papa sufren un fenómeno conocido como indución al endulzamiento en frío, según el cual se acelera el rango de conversión al almidón para reducir azúcares (ej., glucosa y fructosa). Cuando se rebanan las papas crudas y se cocinan en aceite a altas temperaturas, los azúcares reductores acumulados reaccionan liberando aminoácidos en la célula de la papa, formando inaceptables pigmentaciones marrones a negras en las papas en hojuelas o fritas, debido a una reacción no enzimática del tipo Maillard. Las plantas procesadoras, generalmente no aceptan comprar papas con estos colores. Todos los cultivares comerciales de papa usados para la producción de hojuelas y papas fritas acumulan excedentes de azúcares reductores libres al ser expuestos al estrés del frío. Si una “papa procesada en frío” está disponible, la energía ahorrada puede ser aprovechada en aquellas regiones de crecimiento de papa donde las temperaturas de almacenamiento exterior son bajas. En las regiones donde las temperaturas son ligeramente altas, pueden incrementarse los costos de refrigeración. Sin embargo, este gasto se compensaría al eliminarse la necesidad de comprar químicos que producen dormancia, y los que sirven para controlar enfermedades y mejorar la calidad del tubérculo en el largo plazo. La meta primaria de esta revisión es describir la investigación reciente de naturaleza bioquímica y molecular relacionada con los mecanismos que regulan la poscosecha y el endulzamiento inducido en frío en los tubérculos de papa. No se ha hecho ningún esfuerzo para explicar la investigatión realizada sobre la manipulación genética del metabolismo del carbono entre el almidón y los azúcares libres durante la fotosíntesis y/o durante desarrollo de la papa respecto a las interacciones de la fuente.
Similar content being viewed by others
Abbreviations
- Aclnv:
-
acid invertase
- AGPase:
-
adenosine diphosphate glucose pyrophosphorylase
- ATP-PFK:
-
adenosine triphosphate dependent fructose-6-phosphate 1-phosphotransferase
- FBPase:
-
fructose-l,6-bisphosphatase
- Fru-1,6-P2 :
-
fructose-l,6-bisphosphate
- Fru-2,6-P2 :
-
fractose-2,6-bisphos-phate
- Fru-6-P:
-
fructose-6-phosphate
- GFP:
-
glucose forming potential
- Glc-l-P:
-
glucose-1-phosphate
- Glc-6-P:
-
glucose-6-phosphate
- PEP:
-
phos-phoenolpyruvate
- PK:
-
pyruvate kinase
- PPi:
-
inorganic pyrophosphate
- PPi-PFK:
-
pyrophosphate dependent fructose-6-phosphate 1-phosphotransferase
- QTL:
-
quantitative trait locus
- RT-PCR:
-
reverse transcriptase polymerase chain reaction
- UDP-Glc:
-
uridine diphosphate glucose
- UGPase:
-
uridine diphosphate glucose pyrophosphorylase
- SPS:
-
sucrose phosphate synthase
Literature cited
Amir, J., V. Kahn, and M. Unterman. 1977. Respiration, ATP level, and sugar accumulation in potato tubers during storage at 4 C. Phytochemistry 16:1495–1498.
ap Rees, T. 1974. Pathways of carbohydrate breakdown in plants, MTP International Review of Science. Plant Biochemistry 11:89–127.
ap Rees, T., W.L. Dixon, C.J. Pollock, and F. Franks. 1981. Low temperature sweetening of higher plants.In: Rhodes, M.J.C. (ed.), Recent advances in the biochemistry of fruits and vegetables. Academic Press, New York, pp.41–61.
ap Rees, T., M.M. Burrell, T.G. Entwistle, J.B.W. Hammond, D. Kirk, and N.J. Kruger. 1988. Effects of low temperature on the respiratory metabolism of carbohydrate by plants.In: Woodward, I.F., and S.P. Long (eds.), Plants and Temperature. Company of Biologists, Cambridge, UK. pp.377–393.
ap Rees, T. and S. Morrell. 1990. Carbohydrate metabolism in developing potatoes. Am Potato J 67:835–847.
Barichello, V., R.Y. Yada, R.H. Coffin, and D.W. Stanley. 1990a Low temperature sweetening in susceptible and resistant potatoes: Starch structure and composition. J Food Sci 55:1054–1059.
Barichello, V., R.Y. Yada, R.H. Coffin, and D.W. Stanley. 1990b. Respiratory enzyme activity in low temperature sweetening of susceptible and resistant potatoes. J Food Sci 55:1060–1063.
Borovkov, A.Y., P.E. McClean, J.R. Sowokinos, S.H. Ruud, and G.A. Secor. 1996 Effect of expression of UDP-Glucose pyrophosphorylase antisense and ribozyme RNAs on the enzyme activity and carbohydrate composition of transgenic potato plants. J Plant Physiol 147:644–652.
Bredemeijer, G.M.M., H.C.J. Burg, P.A.M. Claassen, and W.J. Stiekema 1991. Phosphofructokinase in relation to sugar accumulation in cold-stored potato tubers. J. Plant Physiol 138:129–135.
Bryce, J.H., and S.A. Hill. 1993. Energy production in plant cells.In: Lee, P.J., and R.C. Leegood (eds.), Plant Biochemistry and Molecular Biology. Wiley, Chichester. pp. 1–21.
Burrell, M.M. 1994. Control of carbohydrate metabolism in potato tubers.In: Belknap, W.R., M.E. Vayda, and W.P. Park (eds.), The Molecular and Cellular Biology of the Potato, 2nd edition, C.A.B. International, Wallingford, UK. pp.45–55.
Burrell, M.M., P.J. Mooney, M. Blundy, D. Carter, F. Wilson, J. Green, K.S. Blundy, and T. ap Rees. 1994. Genetic manipulation of 6- phosphofructokinase in potato tubers. Planta 194:95–101.
Burton, W.G. 1969. The sugar balance in some British potato varieties during storage, II. The effects of tuber age, previous storage temperature, and intermittent refrigeration upon low-temperature sweetening. Eur Potato J 12:81–95.
Claassen, P.A.M., and M.A.W. Budde. 1996. Possible involvement of fructose 1,6-bisphosphatase in cold-induced sweetening in potatoes. Potato Res 39:141–151.
Claassen, P.A.M., M.A.W. Budde, and M.H. van Calker. 1993. Increase in phosphorylase activity during cold-induced sugar accumulation in potato tubers. Potato Res36:205–217.
Claassen, P.A.M., M.A.W. Budde, H.J. de Ruyter, M.H. van Calker, and A. van Es. 1991. Potential role of pyrophosphate:fructose 6-phos-phate phosphotransferase in carbohydrate metabolism of cold stored tubers ofSolanum tuberosum cv. Bintje. Plant Physiol 95:1243–1249.
Cochrane, M.P., C.M. Duffus, M.J. Allison, G.R. Mackay. 1991. Amylolytic activity in stored potato tubers. 2. The effect of low-temperature storage on the activities of a- and ß-amylase and α-glucosidase in potato tubers. Potato Res 34:333–341.
Coffin, R.H., R.Y. Yada, K.L. Parkin, B. Grodzinski, and D.W. Stanley. 1987. Effect of low temperature storage on sugar concentrations and chip color of certain processing potato cultivars and selections. J Food Sci 52:639–645.
Coleman, W.K., G.C.C. Tai, S. Clayton, M. Howie, and A. Pereira 1993. A portable monitor for the rapid assessment of processing quality of stored potato tubers. Am Potato J 70:909–923.
Copeland, L., and J.F. Turner. 1987. The regulation of glycolysis and the pentose phosphate pathway.In: Davies D.D. (ed.), The Biochemistry of Plants. Academic Press, New York. pp. 107–128.
Cottrell, J.E., C.M. Duffus, L. Paterson, G.R. Mackay, M.J. Allison, and H. Bain. 1993. The effect of storage temperature on reducing sugar concentration and the activities of three amylolytic enzymes in tubers of the cultivated potato,Solarium tuberosum L. Potato Res 36:107–117.
Davies, H.V. 1990. Carbohydrate metabolism during sprouting. Am Potato J 67:815–827.
Davies, H.V., R.A. Jefferies, and L. Scobie. 1989. Hexose accumulation in cold stored tubers of potatoSolanum tuberosum L). The effects of water stress. J. Plant Physiol 134:471–475.
Deiting, U., R. Zrenner, and M. Stitt. 1998. Similar temperature requirement for sugar accumulation and for the induction of new forms of sucrose phosphate synthase and amylase in cold-stored potato tubers. Plant, Cell Env 21:127–138.
Dixon, W.L., and T. ap Rees. 1980a. Identification of the regulatory steps in glycolysis in potato tubers. Phytochemistry 19:1297–1301.
Dixon, W.L., and T. ap Rees. 1980b. Carbohydrate metabolism during cold-induced sweetening of potato tubers. Phytochemistry 19:1653–1656.
Dixon, W.L., F. Franks, and T. ap Rees. 1981. Cold lability of phosphofructokinase from potato tubers. Phytochemistry 20:969–972.
Doehlert, D.C., and S.C. Huber. 1983. Regulation of spinach leaf sucrose phosphate synthase by glucose-6-phosphate, inorganic phosphate and pH. Plant Physiol 73:989–994.
Doucette, M.S., and M.K. Pritchard. 1993. ABA involvement in low temperature sweetening of potatoes. Acta Horticulturae 343:293–294.
Dunford, R. 1992. Pyruvate kinase and glycolytic control in potatoes. Ph.D thesis. Cambridge University, Cambridge, UK.
Ewing, E.E., A.H. Senesac, and J.B. Sieczka. 1981. Effects of short periods of chilling and warming on potato sugar content and chipping quality. Am Potato J 58:633–647.
Geigenberger, P., L. Merlo, R. Reimholz, and M. Stitt. 1994. When growing potato tubers are detached from their mother plant there is a rapid inhibition of starch synthesis, involving inhibition of ADP- glucose pyrophosphorylase. Planta 193:486–493.
Geigenberger, P., R. Reimholz, M. Geiger, L. Merlo, V. Canale, and M. Stitt. 1997. Regulation of sucrose and starch metabolism in potato tubers in response to short-term water deficit. Planta 201:502–518.
Gottlob-McHugh, S.G., R.S. Sangwan, S.D. Blakeley, G.C. Vanlerberghe, K. Ko, D.H. Turpin, W.C. Plaxton, B.L. Miki, and D.T. Dennis. 1992. Normal growth of transgenic tobacco plants in the absence of cytosolic pyruvate kinase. Plant Physiol 100:820–825.
Gounaris, Y., and J.R. Sowokinos. 1992, Two-dimensional analysis of mitochondrial proteins from potato cultivars resistant and sensitive to cold-induced sweetening. J Plant Physiol 140:611–616.
Greiner, S., T. Rausch, U. Sonnewald, and K. Herbers. 1999. Ectopic expression of a tobacco invertase inhibitor homolog prevents cold-induced sweetening of potato tubers. Nat Biotechnol 17:708–711.
Haake, V., R. Zrenner, U. Sonnewald, and M. Stitt. 1998. A moderate decrease of plastid aldolase activity inhibits photosynthesis, alters the level of sugars and starch, and inhibits growth of potato plants. Plant J 14:147–157.
Hajirezaei, M., U. Sonnewald, R. Viola, S. Carlisle, D. Dennis, and M. Stitt. 1994. Transgenic potato plants with strongly decreased expression of pyrophosphate:fructose-6-phosphate phosphotransferase show no visible phenotype and only minor changes in metabolic fluxes in their tubers. Planta 192:16–30.
Hammond, J.B.W., M.M. Burrell, and N.J. Kruger. 1990. Effect of low temperature on the activity of phosphofructokinase from potato tubers. Planta 180:613–616.
Hatch, M.D. and K.T. Glasziou. 1963. Sugar accumulation cycle in sugar cane. II Relationship of invertase activity to sugar content and growth rate in storage tissue of plants grown in controlled environments. Plant Physiol 38:344–348.
Hatzfeld, W-D., J. Dancer, and M. Stitt. 1989. Direct evidence that pyrophosphate:fructose-6-phosphate phosphotransferase can act as a glycolytic enzyme in plants. FEBS Letters 254:215–218.
Hill, L., R. Reimholz, R. Schröder, T.H. Nielsen, and M. Stitt. 1996. The onset of sucrose accumulation in cold-stored potato tubers is caused by an increased rate of sucrose synthesis and coincides with low levels of hexose-phosphates, an activation of sucrose phosphate synthase and the appearance of a new form of amylase. Plant, Cell Env 19:1223–1237.
Hiser, C., and L. Mclntosh. 1990. Alternative oxidase of potato is an integral membrane protein synthesizedde novo during aging of tuber slices. Plant Physiol 93:312–318.
Huber, S.C., and J.L.A. Huber. 1992. Role of sucrose phosphate synthase in sucrose metabolism in leaves. Plant Physiol 99:1275–1278.
Huber, S.C., and J.L.A. Huber. 1996. Role and regulation of sucrose-phosphate synthase in higher plants. Annu Rev Plant Physiol Mol Biol 47:431–444.
Huber, J.L.A., S.C. Huber, and T.H. Nielsen. 1989. Protein phosphorylation as a mechanism for regulation of spinach leaf sucrose-phosphate synthase activity. Arch Biochem Biophys 270:681–690.
Isherwood, A. 1973. Starch-sugar interconversion inSolanum tuberosum. Phytochemistry 12:2579- 2591.
Isherwood, A. 1976. Mechanism of starch-sugar interconversion inSolanum tuberosum. Phytochemistry 15:33–41.
Isherwood, F.A., and M.G.H. Kennedy. 1975. The composition of the expressed sap from cold stored potatoes. Phytochemistry 14:83–84.
Isla, M.I., D.P. Leal, M.A Vattuone, and A.R. Sampietro. 1992. Cellular localization of the invertase, proteinaceous inhibitor and lectin from potato tubers. Phytochemistry 31:1115–1118.
Kacser, H. 1987. Control of metabolism.In: Davies D.D. (ed.), The Biochemistry of Plants. Vol 11, Academic Press, London, pp. 39–67.
Katsube, T., Y. Kazuta, H. Mori, K. Nakano, K. Tanizawa, and T. Fukui. 1990. UDP-glucose pyrophosphorylase from potato tuber: cDNA cloning and sequencing. J Biochem 108:321–326.
Kennedy, M.G.H., and F.A. Isherwood. 1975. Activity of phosphorylase inSolanum tuberosum during low temperature storage. Phytochemistry 14:667–670.
Knowles, N.R., and L.O. Knowles. 1989. Correlations between electrolyte leakage and degree of saturation of polar lipids from aged potatoSolanum tuberosum L.) tuber tissue. Ann Bot 63:331–338.
Krause, K-P., L. Hill, R. Reimholz, T.H. Nielsen, U. Sonnewald, and M. Stitt. 1998. Sucrose metabolism in cold-stored tubers with decreased expression of sucrose phosphate synthase. Plant, Cell Env 21:285–299.
Kruckeberg, A.L., H.E. Neuhaus, R. Feil, L.D. Gottlieb, and M. Stitt. 1989. Decreased-activity mutants of phosphoglucose isomerase in the cytosol and chloroplast ofClarkia xantiana: Impact on massaction ratios and fluxes to sucrose and starch, and estimation of flux control coefficients and elasticity coefficients. Biochem J 261:457–467.
Kruger, N.J., J.B.W. Hammond, and M.M. Burrell. 1988. Molecular characterization of four forms of phosphofructokinase purified from potato tuber. Arch Biochem Biophys 267:690–700.
Kumar, G.N.M., L.O. Knowles, N. Fuller, and N.R. Knowles. 2000. alpha-1,4 glucan phosphorylase activity correlates with senescent sweetening but not low temperature-induced sweetening in potato. Plant Physiol 123(S):126 (abstract no. 596).
Lorberth, R., G. Ritte, L. Willmitzer, and J. Kossmann. 1998. Inhibition of a starch-granule-bound protein leads to modified starch and repression of cold sweetening. Nat Biotechnol 16:473–477.
Mares, D.J., J.R. Sowokinos, and J.S. Hawker. 1985. Carbohydrate metabolism in developing potato tubers.In: Li, P.H.(ed.), Potato Physiology. Academic Press, New York, pp.279–327.
Merlo, L., P. Geigenberger, M. Hajirezaei, and M. Stitt. (1993) Changes of carbohydrates, metabolites and enzyme activities in potato tubers during development, and within a single tuber along a stolon-apex gradient. J Plant Physiol 142:392–402.
Morrell, S., and T. ap Rees. 1986a. Control of the hexose content of potato tubers. Phytochemistry 25:1073–1076.
Morrell, S., and T. ap Rees. 1986b. Sugar metabolism in developing tubersof Solanum tuberosum. Phytochemistry 25:1579–1585.
Müller-Róber, B., U. Sonnewald, and L. Willmitzer. 1992. Inhibition of the ADP-glucose pyrophosphorylase in transgenic potatoes leads to sugar-storing tubers and influences tuber formation and expression of tuber storage protein genes. EMBO J 11:1229–1238.
Müller-Thurgau, H. 1882. Uber Zuckeranhaufung in Pflanzentheilen in Folge niederer Temperatur. Landwirtsch Jahrb 11:751–828.
Nakae, T. 1971. Multiple forms of uridine diphosphate glucose pyrophosphorylase fromSalmonella typhimurium. J Biol Chem 246:4404–4411.
Nantes, I.L., M.M. Fagian, R. Catisti, P. Arruda, I.G. Maia, and A.E. Vercesi. 1999. Low temperature and aging-promoted expression of PUMP in potato tuber mitochondria. FEBS Letters 457:103–106.
Neuhaus, H.E., A.L. Kruckeberg, R. Feil, and M. Stitt. 1989. Reduced activity of phosphoglucose isomerase in the cytosol and chloroplast ofClarkia xantiana II. Study of the mechanisms which regulate photosynthate partitioning. Planta 178:110–112.
Nielsen, T.H., U. Deiting, and M. Stitt. 1997. A ß-amylase in potato tubers is induced by storage at low temperature. Plant Physiol 113:503–510.
O’Donoghue, E.P., R.Y. Yada, and A.G. Marangoni. 1994. Low temperature sweetening in potato tubers: the role of the amyloplast membrane. J. Plant Physiol 145:335–341.
Pollock, C., and T. ap Rees T. 1975a. Activities of enzymes of sugar metabolism in cold-stored tubers ofSolanum tuberosum. Phytochemistry 14:613–617.
Pollock, C., and T. ap Rees. 1975b. Effect of cold on glucose metabolism by callus and tubers ofSolanum tuberosum. Phytochemistry 14:1903–1906.
Preiss, J. 1982. Regulation of the biosynthesis and degradation of starch. Annu Rev Plant Physiol 33:431–454.
Preiss, J. 1988. Biosynthesis of starch and its regulation.In: Preiss, J. (ed.), The Biochemistry of Plants, Carbohydrates. Vol 14, Academic Press, San Diego, pp. 181–254.
Pressey, R. 1967. Invertase inhibitor from potatoes: Purification, characterization and reactivity with plant invertases. Plant Physiol 42:1780–1786.
Pressey, R. 1969. Role of invertase in accumulation of sugars in cold- stored potatoes. Am Potato J 46:291–297.
Pritchard M.K., and L.R. Adam. 1994. Relationships between fry color and sugar concentration in stored Russet Burbank and Shepody Potatoes. Am Potato J 71:59–68.
Reimholz, R., P. Geigenberger, and M. Stitt. 1994. Sucrose-phosphate synthase is regulated via metabolites and protein phosphorylation in potato tubers, in a manner analogous to the enzyme in leaves. Planta 192:480–488.
Reimholz, R., M. Geiger, V. Haake, U. Deiting, K-P. Krause, U. Sonnewald, and M. Stitt. 1997 Potato plants contain multiple forms of sucrose phosphate synthase, which differ in their tissue distributions, their levels during development, and their responses to low temperature. Plant, Cell Env 20:291–305.
Richardson, D.L., H.V. Davies, H.A. Ross, and G.R. Mackay. 1990. Invertase activity and its relation to hexose accumulation in potato tubers. J Exp Bot 41:95–99.
Samotus, B., M. Niedzwiedz, Z. Kolodziej, M. Leja, and B. Czajkowska. 1974. Storage and reconditioning of tubers of Polish potato varieties and strains I. Influence of storage temperature on sugar level in potato tubers of different varieties and strains. Potato Res 17:64–81.
Sasaki, T., K. Tadokoro, and S. Suziki. 1971. Multiple forms of invertase of potato tuber stored at low temperature. Phytochemistry 10:2047–2050.
Schott, K., S. Borchert, B. Müller-Röber, and H.W. Heldt. 1995. Transport of inorganic phosphate and C3- and C6-sugar phosphates across the envelope membranes of potato tuber amyloplasts. Planta 196:647–652.
Scott, P., A.J. Lange, S.J. Pilkis, and N.J. Kruger. 1995. Carbon metabolism in leaves of transgenic tobacco(Nicotiana tabacum L.) containing elevated fructose-2,6-bisphosphate levels. Plant J 7:461–469.
Shekar, V.C., W.M. Iritani, and J.R. Magnuson. 1979. Starch-sugar inter-conversion inSolanum tuberosum L. II. Influence of membrane permeability and fluidity. Am Potato J 56:225–235.
Sherman, M., and E.E. Ewing. 1983. Effects of temperature and low oxygen atmospheres on respiration, chip color, sugars, and malate of stored potatoes. J Amer Soc Hort Sci 108:129–133.
Smith, A.M., K. Denyer, and C. Martin. 1997. The synthesis of the starch granule. Annu Rev Plant Physiol Plant Mol Biol 48:67–87.
Sonnewald, U., A. Basner, G. Burkhard, and M. Steup. 1995. A second L-type isozyme of potato glucan phosphorylase: cloning, antisense inhibition and expression analysis. Plant Mol Bio 27:567–576.
Sowokinos, J.R. 1990a. Effect of stress and senescence on carbon partitioning in stored potatoes. Am Potato J 67:849–857.
Sowokinos, J.R. 1990b. Stress-induced alterations in carbohydrate metabolism.In: Vayda, M.E., and W.P. Park (eds.), The Molecular and Cellular Biology of the Potato. C.A.B. International, Wallingford, UK pp. 137–158.
Sowokinos, J.R. 1999. Choices for potatoes that chip directly from 42 F without reconditioning. Valley Potato Grower 64(114): 14–17.
Sowokinos, J.R. 2001. Pyrophosphorylase inSolanum tuberosum L.: Allelic and isozyme patterns of UDP-Glucose pyrophosphorylase as a marker for cold-sweetening resistance in potatoes. Am J Potato Res 78:57–64.
Sowokinos, J.R., E.C. Lulai, and J.A. Knoper. 1985. Translucent tissue defects inSolanum tuberosum L. I: Alterations in amyloplast membrane integrity, enzyme activities, sugars and starch content. Plant Physiol 78:489–494.
Sowokinos, J.R., B. Morgan, M. Sleeper, and I. Shea 1989. Establishment of glucose-forming-potential (GFP) as a tool to screen breeding clones for processing potential. RRV Potato Res and Reporting Conference pp. 145-155.
Sowokinos, J.R., and J. Preiss. 1982. Pyrophosphorylases inSolanum tuberosum L. HI. Purification, physical and catalytic properties of ADP-Glucose pyrophosphorylase in potatoes. Plant Physiol 69:1459–1466.
Sowokinos, J.R., C.C. Shock, T.D. Stieber, and E.P. Eldredge. 2000. Compositional and enzymatic changes associated with the sugar-end defect in Russet Burbank potatoes subjected to a single, transient, moisture stress period during early tuber bulking. Am J Potato Res 77:47–56.
Sowokinos, J.R., C.T. Thomas, and M.M. Burrell. 1997. Pyrophosphorylases in potato V. Allelic polymorphism of UDP-Glucose pyrophosphorylase in potato cultivars and its association with tuber resistance to sweetening in the cold. Plant Physiol 113:511–517.
Sowokinos, J.R., J.P. Spychalla, and S.L. Desborough. 1993. Pyrophosphorylases inSolanum tuberosum L. IV. Purification, tissue localization, and physicochemical properties of UDP- Glucose pyrophosphorylase. Plant Physiol 101:1073–1080.
Spychalla, J.P., and S.L. Desborough. 1990. Fatty acids, membrane permeability, and sugars of stored potato tubers. Plant Physiol 94:1207–1213.
Spychalla, J.P., B.E. Scheffler, J.R. Sowokinos, and M.W. Bevan. 1994. Cloning, antisense RNA inhibition, and the coordinated expression of UDP-glucose pyrophosphorylase with starch biosynthesis genes in potato tubers. J Plant Physiol 144:444–453.
Stark, D.M., K.P. Timmerman, G.F. Barry, J. Preiss, and G.M. Kishore. 1992. Regulation of the amount of starch in plant tissues by ADP glucose pyrophosphorylase. Science 258:287–292.
Steup, M. 1990. Starch degrading enzymes,In: Lea, P.J. (ed.), Methods in Plant Biochemistry. Vol 3. Academic Press, London, pp. 103–128.
Stitt, M. 1987. Fructose 2,6-bisphosphate and plant carbohydrate metabolism. Plant Physiol 84:201–204.
Stitt, M. 1990. Fructose 2,6-bisphosphate as regulatory metabolite in plants. Annu Rev Plant Physiol. Mol Biol 41:153–185.
Stitt, M., S.C. Huber, and P. Kerr. 1987. Control of photosynthetic sucrose synthesis.In: Hatch, M.D., and N.K. Boardman (eds.), Biochemistry of Plants. Academic, New York. 10:327–407.
Stitt, M., I. Wilke, R. Feil, and H.W. Heldt. 1988. Coarse control of sucrose phosphate synthase in leaves: alterations of kinetic properties in response to the rate of photosynthesis and the accumulation of sucrose. Planta 174:217–230.
Stitt, M., and U. Sonnewald. 1995. Regulation of metabolism in transgenic plants. Annu Rev Plant Physiol Plant Mol Biol 46:341–368.
Sweetlove, L.J., M.M. Burrell, and T. ap Rees. 1996a. Characterization of transgenic potato(Solanum tuberosum) tubers with increased ADPglucose pyrophosphorylase. Biochem J 320:487–492.
Sweetlove, L.J., M.M. Burrell, and T. ap Rees. 1996b. Starch metabolism in tubers of transgenic potato(Solanum tuberosum) with increased ADPglucose pyrophosphorylase. Biochem J 320:493–498.
Tauberger, E., A.R. Fernie, M. Emmermann, A. Renz, J. Kossmann, L. Willmitzer, and R.N. Trethewey. 2000. Antisense inhibition of plastidial phosphoglucomutase provides compelling evidence that potato tuber amyloplasts import carbon from the cytosol in the form of glucose-6-phosphate. Plant J 23:43–53.
Theologis, A., and G.G. Laties. 1976. Membrane lipid integrity as a prerequisite of cyanide-resistant respiration in potato slices. Plant Physiol 57:S-93.
Thill, C.A. and S.J. Peloquin. 1994. Inheritance of potato chip color at the 24-chromosome level. Am Potato J 71:629–646.
Trevanion, S.J., and N.J. Kruger. 1991. Effect of temperature on the kinetic properties of pyrophosphate:fructose 6-phosphate phos-photransferase from potato tuber. J. Plant Physiol 137:753–759.
Viola, R. and H.V. Davies. 1994. Effect of temperature on pathways of carbohydrate metabolism in tubers of potato(Solanum tuberosum L). Plant Science 103:135–143.
Vliet, W.F. and W.H. vanSchriemer. 1960. The sugar accumulation of potatoes kept at low temperature, as studied in a small section of Dutch varieties. Eur Potato J 3:263–271.
Willlmitzer, L., W.B. Frommer, J. Kossmann, B. Müller-Röber, J. Riesmeier, U. Sonnewald, U-I. Flügge, and H. Heldt. 1994. Transgenic potatoes changed in carbohydrate partitioning and allocation.In: Belknap, W.R., M.E. Vayda, and W.P. Park (eds.), The Molecular and Cellular Biology of the Potato. 2nd Edition, C.A.B. International, Wallingford, UK. pp.57–65.
Workman, M., A. Cameron, and J. Twomey. 1979. Influence of chilling on potato tuber respiration, sugar, o-dihydroxyphenolic content and membrane permeability. Am Potato J 56:277–288.
Yada, R.Y., R.H. Coffin, K.W. Baker, and M.J. Leszkowiat. 1990. An electron microscopic examination of the amyloplast membranes from a potato seedling resistant and a processing potato cultivar susceptible to low temperature sweetening. Can Inst Fd Sci Technol J 23:145–148.
Zhou, D., A. Mattoo, N. Li, H. Imaseki, and T. Solomos. 1994.Plant Gene Register. Complete nucleotide sequence of potato tuber acid invertase cDNA. Plant Physiol 106:397–398.
Zrenner, R., K. Schuler, and U. Sonnewald. 1996. Soluble acid invertase determines the hexose-to-sucrose ratio in cold-stored potato tubers. Planta 198:246–252.
Zrenner, R., L. Willmitzer, and U. Sonnewald. 1993. Analysis of the expression of potato uridinediphosphate-glucose pyrophosphorylase and its inhibition by antisense RNA. Planta 190:247–252.
Author information
Authors and Affiliations
Corresponding author
Additional information
University of Minnesota, Agricultural Experimental Station Scientific Journal Series No: 001210061.
Rights and permissions
About this article
Cite this article
Sowokinos, J.R. Biochemical and molecular control of cold-induced sweetening in potatoes. Am. J. Pot Res 78, 221–236 (2001). https://doi.org/10.1007/BF02883548
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF02883548