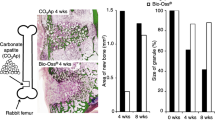
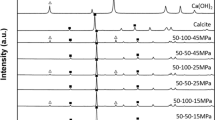
Abstract
Although bone apatite is the carbonate apatite which contains 6–9 mass% carbonate in its apatite structure, sintered hydroxyapatite free from carbonate has been used as artificial bone substitutes since carbonate apatite cannot be sintered due to the thermal decomposition at high temperature required for sintering. We have proposed a two-step fabrication method for fabrication of carbonate apatite block. First step is the fabrication of a precursor block such as calcium carbonate and tricalcium phosphate. Then the precursor block is immersed in solution in which missing elements for the fabrication of carbonate apatite is supplied to the precursor from the solution. Based on the dissolution-precipitation reaction, the composition of the precursor is transformed to carbonate apatite, the inorganic component of the bone. Carbonate apatite thus fabricated was found to upregulate osteoblastic cells’ differentiation process. Osteoclasts resorbed carbonate apatite whereas no resorption pits were observed in the case of sintered hydroxyapatite. As a result, carbonate apatite showed much higher osteoconductivity than sintered hydroxyapatite. Furthermore, carbonate apatite is replaced to the bone when implanted in the bone defect, whereas sintered hydroxyapatite remained in the bone defect keeping its original shape. Therefore, carbonate apatite fabricated based on dissolution-precipitation reaction using a precursor is thought to be one of an ideal bone replacements for the next generation.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Ishikawa K, Matsuya S, Miyamoto Y, Kawate K (2003) Bioceramics. In: Mai YW, Teoh SH (eds) Comprehensive structural integrity, vol 9, Bioengineering. Elsevier, San Diego, pp 169–214
LeGerous RZ (1991) Calcium phosphates in oral biology and medicine. Karger, New York
LeGeros RZ, Ben-Nissan B (2014) Introduction to synthetic and biological apatites. In: Ben-Nissan B (ed) Advances in calcium phosphate biomaterials. Springer, New York, pp 1–18
Elliot JC (1994) Structure and chemistry of the apatites and other calcium orthophosphates. Elsevier, Amsterdam
Ito A, Maekawa K, Tsutsumi S, Ikazaki F, Tateishi T (1997) Solubility product of OH-carbonated hydroxyapatite. J Biomed Mater Res 36:522–528
Okazaki M, Moriwaki Y, Aoba T, Doi Y, Takahashi J (1981) Solubility behavior of CO3 apatites in relation to crystallinity. Caries Res 15:477–483
Ishikawa K, Ishikawa Y, Kuwayama N (1991) Preparation of carbonate-bearing hydroxyapatites and their sintering properties. Chem Exp 6(7):463–466
Aoki H, Kato K, Ogiso M (1971) Studies on the application of apatite to dental materials. J Dent Eng 18:86–89
Jarcho M (1980) Calcium phosphate ceramics as hard tissue prosthetics. Clin Orthop Relat Res 157:259–278
de Groot K (1983) Bioceramics of calcium phosphates. CRC Press, Boca Raton
Sekiya M (1964) Gypsum. Gihodo, Tokyo
Ishikawa K (2010) Bone substitute fabrication based on dissolution-precipitation reaction. Materials 3:1138–1155
Ishikawa K, Matsuya S, Lin X, Zhang L, Yuasa T, Miyamoto Y (2010) Fabrication of low crystalline B-type carbonate apatite block from low crystalline calcite block. J Ceram Soc Jpn 118(5):341–344
Suzuki Y, Matsuya S, Udoh K, Nakagawa M, Tsukiyama Y, Koyano K, Ishikawa K (2005) Fabrication of hydroxyapatite block from gypsum block based on (NH4)2HPO4 treatment. Dent Mater J 24(4):515–521
Lowmunkong R, Sohmura T, Takahashi J, Suzuki Y, Matsuya S, Ishikawa K (2007) Transformation of 3DP gypsum model to HA by treating in ammonium phosphate solution. J Biomed Mater Res Part B App Biomater 80B:386–393
Lowmunkong R, Sohmura T, Suzuki Y, Matsuya S, Ishikawa K (2009) Fabrication of freeform bone-filling calcium phosphate ceramics by gypsum 3D printing method. J Biomed Mater Res B Appl Biomater 90(2):531–539
Wakae H, Takeuchi A, Udoh K, Matsuya S, Munar M, LeGeros RZ, Nakasima A, Ishikawa K (2008) Fabrication of macroporous carbonate apatite foam by hydrothermal conversion of α-tricalcium phosphate in carbonate solutions. J Biomed Mater Res A 87(4):957–963
Maruta M, Matsuya S, Nakamura S, Ishikawa K (2011) Fabrication of low-crystalline carbonate apatite foam bone replacement based on phase transformation of calcite foam. Dent Mater J 30(1):14–20
Nomura S, Tsuru K, Matsuya S, Takahashi I, Ishikawa K (2014) Fabrication of carbonate apatite block from set gypsum based on dissolution-precipitation reaction in phosphate-carbonate mixed solution. Dent Mater J 33(2):166–172
Lee Y, Hahm YM, Matsuya S, Nakagawa M, Ishikawa K (2007) Development of macropores in calcium carbonate body using novel carbonation method of calcium hydroxide/sodium chloride composite. J Mater Sci 42:5728–5735
Lee Y, Hahm YM, Matsuya S, Nakagawa M, Ishikawa K (2007) Characterization of macroporous carbonate-substituted hydroxyapatite bodies prepared in different phosphate solutions. J Mater Sci 42:7843–7849
Otsu A, Tsuru K, Maruta M, Munar ML, Matsuya S, Ishikawa K (2012) Fabrication of microporous calcite block from calcium hydroxide compact under carbon dioxide atmosphere at high temperature. Dent Mater J 31(4):593–600
Monma H, Kanazawa T (1976) The hydration of α-tricalcium phosphate. Yogyo Kyokai-Shi 84:209–213
Ishikawa K (2008) Calcium phosphate cement. In: Kokubo T (ed) Handbook of bioceramics and their application. CRC Press, Boca Raton, pp 464–484
Nagai H, Fujioka-Kobayashi M, Fujisawa K, Ohe G, Takamaru N, Hara K, Uchida D, Tamatani T, Ishikawa K, Miyamoto Y (2015) Effects of low crystalline carbonate apatite on proliferation and osteoblastic differentiation of human bone marrow cells. J Mater Sci Mater Med 26(2):99–107
To be submitted
Nguyen XTT, Maruta M, Tsuru K, Matsuya S, Ishikawa K (submitted) Fabrication and histological study of ant colony type carbonate apatite foam. Biomaterials
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer International Publishing Switzerland
About this entry
Cite this entry
Ishikawa, K. (2016). Carbonate Apatite Bone Replacement. In: Antoniac, I. (eds) Handbook of Bioceramics and Biocomposites. Springer, Cham. https://doi.org/10.1007/978-3-319-12460-5_8
Download citation
DOI: https://doi.org/10.1007/978-3-319-12460-5_8
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-319-12459-9
Online ISBN: 978-3-319-12460-5
eBook Packages: Chemistry and Materials ScienceReference Module Physical and Materials ScienceReference Module Chemistry, Materials and Physics