Abstract
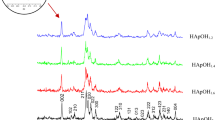
Bone mineral of human is different in composition from the stoichiometric hydroxyapatite (Ca10(PO4)6(OH)2) in that it contains additional ions, of which CO 2−3 is the most abundant species. Carbonate-substituted hydroxyapatite (CHA) bodies were prepared by the hydrothermal treatment of highly porous calcium carbonate (CaCO3) body at 120 °C in 1 M M2HPO4 and M3PO4 solutions (M = NH4 or K). It was found that CaCO3 body was almost transformed into CHA body after hydrothermal treatment for 24 h irrespective of type of phosphate solution. However, a small amount of CaCO3 still remained after the treatment in K3PO4 for 48 h. Crystal shape of CHA bodies prepared in those solutions except for K2HPO4 was flake-like, which was different from that (stick-like) of original CaCO3 body used for the preparation of CHA body. CHA prepared in the K2HPO4 showed globule-like crystal. Average pore size and hole size of the CHA bodies were 150, 70 μm and their porosities were about 89% irrespective of the solution. Carbonate content was slightly higher in the CHA bodies obtained from potassium phosphate solutions than in those obtained from ammonium phosphate solutions. Mostly B-type CHA was obtained after the hydrothermal treatment in the potassium phosphate solutions. On the other hand, mixed A- and B-type CHA (ca. 1–2 in molar ratio) was obtained in the ammonium phosphate solutions. The content of CO 2−3 in the CHA body depended on the type of phosphate solution and was slightly larger in the potassium phosphate solutions.



Similar content being viewed by others
References
Rau JV, Cesaro SN, Ferro D, Barinov SM, Fadeeva JV (2004) J Biomed Mater Res Part B: Appl Biomater 71B(2):441
Baig AA, Fox JL, Su J, Wang Z, Otsuka M, Higuchi WI, Legeros RZ (1996) J Colloid Interface Sci 179:608
Tang R, Henneman ZJ, Nancollas GH (2003) J Cryst Growth 249:614
Rieters IY, Maeyer EAPD, Verbeeck RMH (1996) Inorg Chem 35:5791
Wenk HR, Heidelbach F (1999) Bone 24(4):361
Landi E, Tampieri A, Celotti G, Langenati R, Sandri M, Sprio S (2005) Biomaterials 26:2835
Barralet J, Best S, Bonfield W (1998) J Biomed Mater Res 41(1):79
Barralet J, Akao M, Aoki H (2000) J Biomed Mater Res 49(2):176
Suchanek WL, Shuk P, Byrappa K, Riman RE, Tenhuisen KS, Janas VF (2002) Biomaterials 23:699
Redey SA, Nardin M, Assolant DB, Rey C, Delannoy P, Sedel L, Marie PJ (2000) J Biomed Mater Res 50(3):353
Barralet JE, Aldred S, Wright AJ, Coombes AGA (2002) J Biomed Mater Res 60:360
Matsumoto T, Okazaki M, Inoue M, Ode S, Chien CC, Nakao H, Hamada Y, Takahashi J (2002) J Biomed Mater Res 60:651
Barralet JE, Best SM, Bonfield W (2000) J Mater Sci: Mater Med 11:719
Tonsuaadu K, Peld M, Leskela T, Mannonen R, Niinisto L, Veiderma M (1995) Thermochim Acta 256:55
Feki HE, Savariault JM, Salah AB, Jemal M (2000) Solid State Sci 2:577
Oliverira LM, Rossi AM, Lopes RT (2000) Appl Radiat Isotopes 52:1093
Fleet ME, Liu X (2004) J Solid State Chem 177:3174
Fleet ME, Liu X, King PL (2004) Am Mineral 89:1422
Landi E, Tampieri A, G Celotti, Vichi L, Sandri M (2004) Biomaterials 25:1763
Fleet ME, Liu X (2003) J Solid State Chem 174:412
Redey SA, Razzouk S, Rey C, Assollant DB, Leroy G, Nardin M, Cournot G (1999) J Biomed Mater Res 45:140
Ellies LG, Lelson DGA, Featherstone JDB (1988) J Biomed Mater Res 22:541
Navarro M, Valle SD, Martinez S, Zeppetelli S, Ambrosio L, Planell JA, Ginebra MP (2004) Biomaterials 25:4233
Verveecke G, Lemaitre J (1990) J Cryst Growth 104:820
Legeros RZ, Trautz OR (1967) Science 155:1409
Legeros RZ (1991) In: Meyers HM (ed) Monographs in oral science, vol 15. KARGER, Basel, p 18
Bonel G, Montel G (1964) Comp Rend Acad Sci (Paris) 258:923
Legeros RZ (1991) In: Meyers HM (ed) Monographs in oral science, vol 15. KARGER, Basel, p 89
Cullity BD (1978) Elements of X-ray Diffraction, 2nd edn. Addison-Wesley Pub Co Inc, Philippines, p 102
Bouhaouss A, Bensaoud A, Laghzizil A, Ferhat M (2001) Int J Inorg Mater 3:437
Dekker RJ, Bruijn JDD, Stigter M, Barrere F, Layrolle P, Blitterswijk CAV (2005) Biomaterials 26:5231
Elliott JC (1994) Structure and chemistry of the apatites and other calcium orthophosphates. Studies in Inorganic Chemistry 18, Elsevier, Amsterdam, pp 230–234
Acknowledgements
This study was supported in part by a Grant-in-aid for Scientific Research from the Ministry of Education, Sports, Culture, Science, and Technology, Japan
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lee, Y., Hahm, Y.M., Matsuya, S. et al. Characterization of macroporous carbonate-substituted hydroxyapatite bodies prepared in different phosphate solutions. J Mater Sci 42, 7843–7849 (2007). https://doi.org/10.1007/s10853-007-1629-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-007-1629-3