Abstract
Mesenchymal stromal/stem cells (MSC) are promising candidates for the development of cell-based therapies for various diseases and are currently being evaluated in a number of clinical trials (Sharma et al., Transfusion 54:1418–1437, 2014; Ikebe and Suzuki, Biomed Res Int 2014:951512, 2014). MSC for therapeutic applications are classified as advanced therapy medicinal products (ATMP) (Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004) and must be prepared according to good manufacturing practices (http://ec.europa.eu/health/documents/eudralex/vol-4). They may be derived from different starting materials (mainly bone marrow (BM), adipose tissue, or cord blood) and applied as fresh or cryopreserved products, in the autologous as well as an allogeneic context (Sharma et al., Transfusion 54:1418–1437, 2014; Ikebe and Suzuki, Biomed Res Int 2014:951512, 2014; Sensebé and Bourin, Transplantation 87(9 Suppl):S49–S53, 2009). In any case, they require an approved and well-defined panel of assays in order to be released for clinical use.
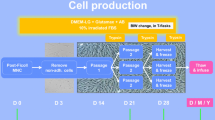
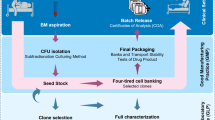
This chapter describes analytical methods implemented and performed in our cell factory as part of the release strategy for an ATMP consisting of frozen autologous BM-derived MSC. Such methods are designed to assess the safety (sterility, endotoxin, and mycoplasma assays) and identity/potency (cell count and viability, immunophenotype and clonogenic assay) of the final product. Some assays are also applied to the biological starting material (sterility) or carried out as in-process controls (sterility, cell count and viability, immunophenotype, clonogenic assay).
The validation strategy for each analytical method is described in the accompanying Chapter 20.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Sharma RR, Pollock K, Hubel A et al (2014) Mesenchymal stem or stromal cells: a review of clinical applications and manufacturing practices. Transfusion 54:1418–1437
Ikebe C, Suzuki K (2014) Mesenchymal stem cells for regenerative therapy: optimization of cell preparation protocols. Biomed Res Int 2014:951512
Sensebé L, Bourin P (2009) Mesenchymal stem cells for therapeutic purposes. Transplantation 87(9 Suppl):S49–S53
Regulation (EC) No 1394/2007 of the European Parliament and of the Council of 13 November 2007 on advanced therapy medicinal products and amending Directive 2001/83/EC and Regulation (EC) No 726/2004
EudraLex – volume 4 – good manufacturing practice (GMP) guidelines – part I – basic requirements for medicinal products. http://ec.europa.eu/health/documents/eudralex/vol-4
Guideline on human cell-based medicinal products. EMEA/CHMP/410869/2006
Rayment EA, Williams DJ (2010) Concise review: mind the gap: challenges in characterizing and quantifying cell- and tissue-based therapies for clinical translation. Stem Cells 28:996–1004
EudraLex – volume 4 – good manufacturing practice (GMP) guidelines – annex 1 – manufacture of sterile medicinal products. http://ec.europa.eu/health/documents/eudralex/vol-4
Gnecchi M, Melo LG (2009) Bone marrow-derived mesenchymal stem cells: isolation, expansion, characterization, viral transduction, and production of conditioned medium. Methods Mol Biol 482:281–294
Wagey R, Short B (2013) Isolation, enumeration, and expansion of human mesenchymal stem cells in culture. Methods Mol Biol 946:315–334
Roseti L, Serra M, Bassi A (2015) Standard operating procedure for the good manufacturing practice-compliant production of human bone marrow mesenchymal stem cells. Methods Mol Biol 1283:171–186
Guideline on potency testing of cell based immunotherapy medicinal products for the treatment of cancer, EMEA/CHMP/410869/2006
Wuchter P, Bieback K, Schrezenmeier H et al (2015) Standardization of good manufacturing practice-compliant production of bone marrow-derived human mesenchymal stromal cells for immunotherapeutic applications. Cytotherapy 17:128–139
Dominici M, Le Blanc K, Mueller I et al (2006) Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8:315–317
Commission Directive 2006/17/EC implementing Directive 2004/23/EC of the European Parliament and of the Council as regards certain technical requirements for the donation, procurement and testing of human tissues and cells
Directive 2004/23/EC of the European Parliament and of the Council on setting standards of quality and safety for the donation, procurement, testing, processing, preservation, storage and distribution of human tissues and cells
European Pharmacopoeia 8.0, Section 2.6.27 (Microbiological control for cellular products) (2014) European Directorate for the Quality of Medicines & HealthCare, Strasbourg, FR
European Pharmacopoeia 8.0, Section 2.6.1 (Sterility) (2014)
European Pharmacopoeia 8.0, Section 2.6.14 (Bacterial endotoxins) (2014) European Directorate for the Quality of Medicines & HealthCare, Strasbourg, FR
Endosafe – PTS Product Validation (2007) Endosafetimes – volume 13, n. 1 – Charles River Laboratories
European Pharmacopoeia 8.0, Section 2.6.7 (Mycoplasmas) (2014) European Directorate for the Quality of Medicines &HealthCare, Strasbourg, FR
European Pharmacopoeia 8.0, Section 2.7.24 (Flow Cytometry) (2010) European Directorate for the Quality of Medicines &HealthCare, Strasbourg, FR
US Department of Health (2008), Guidance for FDA reviewers and sponsors: content and review of chemistry, manufacturing, and control (CMC) information for human gene therapy investigational new drug applications (INDs). US Department of Health, FDA, CBER
Castro-Malaspina H, Ebell W, Wang S (1984) Human bone marrow fibroblast colony-forming units (CFU-F). Prog Clin Biol Res 154:209–236
Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3B
Acknowledgments
This work was supported by the Fondazione Cardiocentro Ticino—Lugano. The authors would like to acknowledge Maura Filippini for her excellent editing assistance.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2016 Springer Science+Business Media New York
About this protocol
Cite this protocol
Radrizzani, M., Soncin, S., Lo Cicero, V., Andriolo, G., Bolis, S., Turchetto, L. (2016). Quality Control Assays for Clinical-Grade Human Mesenchymal Stromal Cells: Methods for ATMP Release. In: Gnecchi, M. (eds) Mesenchymal Stem Cells. Methods in Molecular Biology, vol 1416. Humana Press, New York, NY. https://doi.org/10.1007/978-1-4939-3584-0_19
Download citation
DOI: https://doi.org/10.1007/978-1-4939-3584-0_19
Published:
Publisher Name: Humana Press, New York, NY
Print ISBN: 978-1-4939-3582-6
Online ISBN: 978-1-4939-3584-0
eBook Packages: Springer Protocols