Abstract
The key to successful transformation of American chestnut is having the correct combination of explant tissue, selectable markers, a very robust DNA delivery system, and a reliable regeneration system. The most important components of this transformation protocol for American chestnut are the following: starting out with rapidly dividing somatic embryos, treating the embryos gently throughout the Agrobacterium inoculation and cocultivation steps, doing the cocultivation step in desiccation plates, and finally transferring the embryos into temporary-immersion bioreactors for selection. None of these departures from standard Agrobacterium transformation protocols is sufficient by itself to achieve transgenic American chestnut, but each component makes a difference, resulting in a highly robust protocol.
The average transformation efficiency that can be expected using the described protocol is approximately 170 stable embryogenic transformation events per gram of somatic embryo tissue, a considerable improvement over the 20 transformation events per gram we reported in 2006 (Maynard et al. American chestnut (Castanea dentata (Marsh.) Borkh.) Agrobacterium protocols, 2nd ed., 2006). We have regenerated nearly 100 of these events, containing 23 different gene constructs, into whole plants. As of the fall of 2013, we had a total of 1,275 transgenic chestnut trees planted at eight locations in New York State and one in Virginia. Based on a combination of field-trial inoculations, greenhouse small-stem inoculations, and detached-leaf assays, we have identified three transgenes that produce stronger resistance to chestnut blight than non-transgenic American chestnut. Depending on the transgene and the event, this resistance can be either intermediate between American chestnut and Chinese chestnut, approximately equal to or even higher than the resistance naturally found in Chinese chestnut.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Russell EWB (1987) Pre-blight distribution of Castanea dentata (Marsh.) Borkh. Bull Torrey Bot Club 114(2):183–190
Murrill WA (1906) A serious chestnut disease. J NY Bot Garden 7(8):143–153
Diller JD, Clapper RB (1965) A progress report on attempts to bring back the chestnut tree in the eastern United States, 1954-1964. J Forestry 63(3):186–188
Gonzales ML, Vieitez AM, Vieitez E (1985) Somatic embryogenesis from chestnut cotyledon tissue cultured in vitro. Sci Hortic 27:97–103
Piagnani C, Eccher T (1990) Somatic embryogenesis in chestnut. Acta Hortic 280:159–161
Vieitez FJ, San Jose MC, Ballester A, Vieitez AM (1990) Somatic embryogenesis in cultured immature zygotic embryos in chestnut. J Plant Physiol 136:253–256
Merkle SA, Wiecko AT, Watson-Pauley BA (1991) Somatic embryogenesis in American chestnut. Can J For Res 21:1698–1701
Maynard CA (1991) Using PCR to confirm Agrobacterium transformation of American chestnut (Castanea dentata). Abstract No. 927. In: Program and abstracts of the third international congress of plant molecular biology: molecular biology of plant growth and development. Tucson, Arizona. 6–11 Oct 1991
Merkle S, Carraway DT, Watson-Pauley BA, Wilde HD (1992) Somatic embryogenesis and gene transfer in American chestnut. Proceedings of the international chestnut conference, Morgantown, West Virginia, 10–14 July 1992. West Virginal University Press, Morgantown, W.Va
Carraway DT, Wilde HD, Merkle SA (1994) Somatic embryogenesis and gene transfer in American chestnut. J Am Chest Found 8(1):29–33
Vieitez AM, Ballester A, Vieitez ML, Vieitez E (1983) In vitro plantlet regeneration of mature chestnut. J Hortic Sci 58:457–463
Vieitez AM, Vieitez ML (1983) Castanea sativa plantlets proliferated from axillary buds cultivated in vitro. Sci Hortic 18:343–351
Chauvin JE, Salesses G (1988) Advances in chestnut micropropagation (Castanea sp.). Acta Hortic 227:340–345
Chevre AM, Salesses G (1987) Choice of explants for chestnut micropropagation. Acta Hortic 212:517–523
Mullins KV (1987) Micropropagation of chestnut (Castanea sativa Mill.). Acta Hortic 212:525–530
Piagnani C, Eccher T (1988) Factors affecting the proliferation and rooting of chestnut in vitro. Acta Hortic 227:384–386
Strullu DG, Grellier B, Marciniak D, Letouze R (1986) Micropropagation of chestnut and conditions of mycorrhizal synthesis in vitro. New Phytol 102:95–101
Vieitez AM, Ballester A, Vieitez E (1987) Vitrification in chestnut shoots regenerated in vitro. Acta Hortic 212:231–234
Maynard C, Satchwell M, Rieckermann H (1993) Micropropagation of American chestnut (Castanea dentata (Marsh.) Borkh.): rooting and acclimatization. In: Proceedings of the second northern forest genetics association conference, 29–30 July 1993, St. Paul, Minnesota. pp 161–170
Merkle S, Andrade G, Pettis S, Johnson S, Kormanik T, Le H, Maner L (2008) Recent advances in American chestnut in vitro propagation. J Am Chest Found 22:23–30
Carraway DT, Merkle SA (1997) Plantlet regeneration from somatic embryos of American chestnut. Can J For Res 27:1805–1812
Robichaud RL, Lessard VC, Merkle SA (2004) Treatments affecting maturation and germination of American chestnut somatic embryos. J Plant Physiol 161:957–969
Seabra RC, Pais MS (1998) Genetic transformation of European chestnut. Plant Cell Rep 17:177–182
Corredoira E, Montenegro D, San-Jose MC, Vieitez AM, Ballester A (2004) Agrobacterium-mediated transformation of European chestnut embryogenic cultures. Plant Cell Rep 23:311–318
Maynard CA, Polin LD, LaPierre S, Rothrock RE, Powell WA (2006) American chestnut (Castanea dentata (Marsh.) Borkh.) (Chapter 22). In: Wang K (ed) Agrobacterium protocols, 2nd edn. Humana, Totowa, pp 239–251
Polin LD, Liang H, Rothrock R, Nishii M, Diehl D, Newhouse A, Nairn J, Powell WA, Maynard CA (2006) Transformation of American chestnut (Castanea dentata (Marsh.) Borkh.) somatic embryos. Plant Cell Tiss Org Cult 84:69–78
Gisele M, Campbell A, Nairn J, Huong TL, Merkle SA (2009) Sexually mature transgenic American chestnut trees via embryogenic suspension-based transformation. Plant Cell Rep 28:1385–1397
Kong L, Holtz CT, Nairn CJ, Houke H, Powell WA, Baier K, Merkle SA (2013) Application of airlift bioreactors to accelerate genetic transformation in American chestnut. Plant Cell Tiss Org Cult (PCTOC) 117:39–50
Zhang B, Newhouse AE, McGuigan LD, Maynard CA, Powell WA (2011) Agrobacterium-mediated co-transformation of American chestnut (Castanea dentata) somatic embryos with a wheat oxalate oxidase gene. (Extended abstract for the IUFRO meeting in 2011). BioMed Central (BMC) Proc 5(Suppl 7):43
Powell WA, Morley P, King M, Maynard CA (2007) Small stem chestnut blight resistance assay. J Am Chest Found 21(2):34–38
Newhouse AE, Spitzer JE, Maynard CA, Powell WA (2014) Leaf inoculation assay as a rapid predictor of chestnut blight susceptibility. Plant Dis 98(1)
Zhang B, Oakes AD, Newhouse AE, Baier KM, Maynard CA, Powell WA (2013) A threshold level of oxalate oxidase transgene expression reduces Cryphonectria parasitica—induced necrosis in a transgenic American chestnut (Castanea dentata) leaf bioassay. Transgenic Res 22(5):973–982, http://link.springer.com/article/10.1007%2Fs11248-013-9708-5#
Newhouse AE, Zhang B, Northern L, Maynard CA, Powell WA (2010) Analysis of transgenic American chestnut. Phytopathology 100:S89
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for co-transformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
McGuigan L, Northern LC, Stewart KE, Powell WA, Maynard CA (2012) Transforming American chestnut somatic embryos using a temporary-immersion bioreactor system. Poster presented at the Fifth International Chestnut Symposium, 4–8 Sep 2012, National Conservation Training Center, Shepherdstown, WV
Cheng M, Hu T, Layton J, Liu C, Fry JE (2003) Desiccation of plant tissues post-Agrobacterium infection enhances T-DNA delivery and increases stable transformation efficiency in wheat. In Vitro Cell Dev Biol Plant 39:595–604
Hoagland DR, Arnon DI (1950) The water-culture method for growing plants without soil, vol Circ. 347. Univ. of Calif. Agric. Exp. Station, Berkley
Hood EE, Gelvin SB, Melchers LS, Hoekema A (1993) New Agrobacterium helper plasmids for gene transfer to plants. Transgenic Res 2:208–218
White J, Chang SY, Bibb MJ (1990) A cassette containing the bar gene of Streptomyces hygroscopicus: a selectable marker for plant transformation. Nucleic Acids Res 18:1062
Garbino JE, Belknap WR (1994) Isolation of a ubiquitin-ribosomal protein gene (ubi3) from potato and expression of its promoter in transgenic plants. Plant Mol Biol 24:119–127
Haseloff J, Siemering KR (1998) The uses of GFP in plants. In: Chalfie M, Kain S (eds) Green fluorescent protein: properties, applications, and protocols. Wiley-Liss, New York, pp 191–220
Dratewka-Kos E, Rahman S, Grzekzak ZF, Kennedy TD, Murray RK, Lane G (1989) The polypeptide structure of germin as deduced from cDNA sequencing. J Biol Chem 264:4896–4900
Mason HS, DeWald DB, Mullet JE (1993) Identification of a methyl jasmonate-responsive domain in the soybean vspB promoter. Plant Cell 5:241–251
Acknowledgments
Financial support was provided by the Forest Health Initiative, the Monsanto Fund, the American Chestnut Foundation (New York chapter and National), USDA-Biotechnology Risk Assessment Grant program (BRAG), the Consortium for Plant Biotechnology Research (CPBR), and ArborGen LLC.
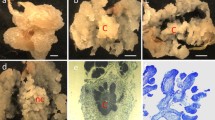
Photography: Greg Boyd Fig. 1; Linda McGuigan Figs. 2, 3a, b; Andy Newhouse Figs. 3c, d; William Powell Fig. 4.
The following individuals have also contributed biological materials, time, and financial support or, in some other way, have contributed to this publication and to the American Chestnut Research and Restoration Project: Stanley and Arlene Wirsig, Herbert and Jane Darling, Dick Radel, Dale Travis, Bryan Burhans, John Dougherty, Joyce Fry, Mary Lou Rath, Dawn Parks, Maud Hinchee, James Donowick, John Ellis, Scott Merkle, Zizhuo Xing, Sharon and Seth LaPierre, Mike Satchwell, Haiying Liang, Katie Damico, and Kristen Russell.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this protocol
Cite this protocol
Maynard, C.A. et al. (2015). Chestnut, American (Castanea dentata (Marsh.) Borkh.). In: Wang, K. (eds) Agrobacterium Protocols. Methods in Molecular Biology, vol 1224. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1658-0_13
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1658-0_13
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1657-3
Online ISBN: 978-1-4939-1658-0
eBook Packages: Springer Protocols