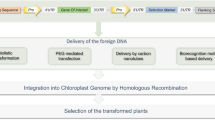
Abstract
As immobile organisms, green plants must be frequently challenged by a broad range of environmental stresses. During these constantly adverse conditions, reactive oxygen species (ROS) levels can rise extremely in plants, leading to cellular dysfunction and cell death presumably due to irreversible protein overoxidation. Once considered merely as deleterious molecules, cells seek to remove them as efficiently as possible. To enhance ROS scavenging capacity, genes encoding antioxidative enzymes can be directly expressed from the genome of plastid (chloroplast), a major compartment for ROS production in photosynthetic organisms. Thus, overexpression of antioxidant enzymes by plastid engineering may provide an alternative to enhance plant’s tolerance to stressful conditions specifically related with chloroplast-derived ROS. Here, we describe basic procedures for expressing glutathione reductase, a vital component of ascorbate-glutathione pathway, in tobacco via plastid transformation technology.
Access this chapter
Tax calculation will be finalised at checkout
Purchases are for personal use only
Similar content being viewed by others
References
Foyer C, Noctor G (2009) Redox regulation in photosynthetic organisms signaling, acclimation, and practical implications. Antioxid Redox Signal 11:861–905
Paulsen CE, Carroll KS (2013) Cysteine-mediated redox signaling: chemistry, biology, and tools for discovery. Chem Rev 113:4633–4679
Waszczak C, Akter S, Jacques S, Huang J, Messens J, Van Breusegem F (2015) Oxidative post-translational modifications of cysteine residues in plant signal transduction. J Exp Bot 66:2923–2934
Smirnoff N, Arnaud D (2019) Hydrogen peroxide metabolism and functions in plants. New Phytol 221:1197–1214
Mhamdi A, Van Breusegem F (2018) Reactive oxygen species in plant development. Development 145:dev164376
Zhang T, Ma M, Chen T, Zhang L, Fan L, Zhang W, Wei B, Li S, Xuan W, Noctor G, Han Y (2020) Glutathione-dependent denitrosation of GSNOR1 promotes oxidative signalling downstream of H2O2. Plant Cell Environ 43:1175–1191
Castro B, Citterico M, Kimura S, Stevens D, Wrzaczek M, Coaker G (2021) Stress-induced reactive oxygen species compartmentalization, perception and signalling. Nat Plants 7:403–412
Foyer CH, Noctor G (2020) Redox homeostasis and signaling in a higher-CO2 world. Annu Rev Plant Biol 71:157–182
Ding H, Wang B, Han Y, Li S (2020) The pivotal function of dehydroascorbate reductase in glutathione homeostasis in plants. J Exp Bot 71:3405–3416
Kerchev P, De Smet B, Waszczak C, Messens J, Van Breusegem F (2015) Redox strategies for crop improvement. Antioxid Redox Signal 23:1186–1205
Le Martret B, Poage M, Shiel K, Nugent GD, Dix PJ (2011) Tobacco chloroplast transformants expressing genes encoding dehydroascorbate reductase, glutathione reductase, and glutathione-S-transferase, exhibit altered anti-oxidant metabolism and improved abiotic stress tolerance. Plant Biotechnol J 9:661–673
Poage M, Le Martret B, Jansen MA, Nugent GD, Dix PJ (2011) Modification of reactive oxygen species scavenging capacity of chloroplasts through plastid transformation. Plant Mol Biol 76:371–384
Wang B, Ding H, Chen Q, Ouyang L, Li S, Zhang J (2019) Enhanced tolerance to methyl viologen-mediated oxidative stress via AtGR2 expression from chloroplast genome. Front Plant Sci 10:1178
Czegeny G, Le Martret B, Pavkovics D, Dix PJ, Hideg E (2016) Elevated ROS-scavenging enzymes contribute to acclimation to UV-B exposure in transplastomic tobacco plants, reducing the role of plastid peroxidases. J Plant Physiol 201:95–100
Bock R (2015) Engineering plastid genomes: methods, tools, and applications in basic research and biotechnology. Annu Rev Plant Biol 66:211–241
Boehm CR, Bock R (2019) Recent advances and current challenges in synthetic biology of the plastid genetic system and metabolism. Plant Physiol 179:794–802
Li S, Chang L, Zhang J (2021) Advancing organelle genome transformation and editing for crop improvement. Plant Commun 2:100141
Daniell H, Jin S, Zhu XG, Gitzendanner MA, Soltis DE, Soltis PS (2021) Green giant—a tiny chloroplast genome with mighty power to produce high-value proteins: history and phylogeny. Plant Biotechnol J 19:430–447
Ahmad N, Michoux F, Lossl AG, Nixon PJ (2016) Challenges and perspectives in commercializing plastid transformation technology. J Exp Bot 67:5945–5960
Wu Y, You L, Li S, Ma M, Wu M, Ma L, Bock R, Chang L, Zhang J (2017) In vivo assembly in Escherichia coli of transformation vectors for plastid genome engineering. Front Plant Sci 8:1454
Motohashi K (2017) Evaluation of the efficiency and utility of recombinant enzyme-free seamless DNA cloning methods. Biochem Biophys Rep 9:310–315
Noctor G, Mhamdi A, Foyer C (2016) Oxidative stress and antioxidative systems: recipes for successful data collection and interpretation. Plant Cell Environ 39:1140–1160
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (32071477, 31700227, and 31300225), Innovation Base for Introducing Talents of Discipline of Hubei Province (2019BJH021), and start-up funding of Anhui Agricultural University.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2022 Springer Science+Business Media, LLC, part of Springer Nature
About this protocol
Cite this protocol
Li, S. et al. (2022). Modification of Chloroplast Antioxidant Capacity by Plastid Transformation. In: Mhamdi, A. (eds) Reactive Oxygen Species in Plants. Methods in Molecular Biology, vol 2526. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2469-2_1
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2469-2_1
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2468-5
Online ISBN: 978-1-0716-2469-2
eBook Packages: Springer Protocols