Abstract
Physarum polycephalum is a protist slime mould that exhibits a high degree of responsiveness to its environment through a complex network of tubes and cytoskeletal components that coordinate behavior across its unicellular, multinucleated body. Physarum has been used to study decision making, problem solving, and mechanosensation in aneural biological systems. The robust generative and repair capacities of Physarum also enable the study of whole-body regeneration within a relatively simple model system. Here we describe methods for growing, imaging, quantifying, and sampling Physarum that are adapted for investigating regeneration and repair.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
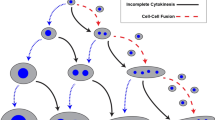
The protist slime mould Physarum polycephalum (which we will refer to as Physarum) exhibits generative capacities that permit the study of the basis of regeneration without the complexities of multi-cellular model systems. Although the multinucleated single-celled slime mould lacks a fixed shape and nervous system, Physarum relays signals throughout its body using a network of branching cytoskeletal tubes that expand and contract to distribute biochemicals (Fig. 1) [1]. That intracellular communication method, known as shuttle streaming, acts as an intrinsic cellular oscillator that drives synchronization across the cell and allows for collective behavior of the organism (Table 1) [1]. The coordination of activities over short and long distances gives rise to more complex mechanosensing, problem solving, and decision-making capabilities that optimize the slime mould’s ability to find food and avoid danger [2,3,4,5,6]. The coordinated oscillations also seem necessary for regeneration and repair capacities [7], however, the prior use of Physarum to study regeneration is limited.
Relatively simple to culture, image, and sample, the single-cell Physarum is capable of regeneration in its most basic form [7]. Physarum can grow from discrete drops of liquid protoplasm to form a single plasmodial cell [7], develop an entire plasmodial network from a small seed point by plasmodial proliferation [8], fuse with separate plasmodial networks [9], and rapidly heal from injuries within its vein network (Table 1). Furthermore, Physarum takes multiple forms that allow hibernation under stressful conditions and growth when environmental conditions are optimal [10]. In suboptimal conditions (low humidity, lack of food, and in the presence of light), Physarum will reconfigure into a dormant, encrusted state known as sclerotia (Table 1) [10], where it can hibernate for months to years. Sclerotia rapidly transforms to a vegetative plasmodial state in the presence of humidity and food sources (Table 1), forming a branching slime mould network . In this dynamic state, Physarum uses shuttle streaming to distribute biochemicals throughout the plasmodial network and is capable of kinase signaling typically observed in eukaryotic organisms [11].
In contrast to regeneration programs that advance toward a large-scale target morphology [12, 13], Physarum has a strong generative capacity with consistent regeneration of hierarchical branching patterns [14]. Similar to Physarum, plants possess a high degree of developmental plasticity, exhibiting consistent, reproducible shoot and root systems from leaf cuttings, with a shoot on one side and a root on the other and small variations in shape from its original body (regeneration in plants fully reviewed in [15]). In this chapter, we define whole-body regeneration as the development of an entire plasmodial network from a small seed point by plasmodial proliferation. In addition, we present methods for transforming sclerotia into a vegetative state and for regeneration of Physarum veins, which are methods for revival and tissue repair, but do not represent regeneration of the entire organism.
Also similar to plants, the large-scale morphology of Physarum is strongly driven by conditions in its local environment, such as attractants, repellants, and substrate stiffness [4, 16]. Physarum is extremely capable of growing and retracting in response to its surroundings and seemingly prioritizes efficient sampling of its surroundings and reinforcement of its beneficial branches over growing into a precise shape [16]. However, given the same environmental conditions, like a maze, Physarum will repeatedly identify the minimum-length solution by retracting veins that reach dead ends [17,18,19]. Therefore, in this chapter, we offer examples of fixed environmental conditions that can be used to assess repair and regrowth in the context of functional patterning, such as the Salt Bridge. Salt interferes with the ionic homeostatic mechanisms of Physarum and therefore exposure to salt will cause Physarum to generate unique whole-body morphologies in its presence [9].
The strong generative capacity of plants relies on stem cells that form at the cut site, elongate and proliferate, and produce new plant bodies [15]. Dissimilar to plants that possess a meristem region of undifferentiated cells, the entire plasmodial Physarum network exists as an undifferentiated single cell. Nuclei of plasmodial Physarum divide without passing through cytokinesis, giving rise to a large multinuclear syncytium [8]. Although plasmodial Physarum and its encrusted state, sclerotia, do not undergo cellular differentiation or even cytokinesis, when Physarum mature or food becomes limited, plasmodia will differentiate into sporangia in the presence of light [11]. The sporangia release haploid spores, which germinate, form flagellated myxamoebae, genetically recombine with myxamoebae from other plasmodia, and fuse into a zygote that develops into a new plasmodium [11].
In this chapter, we present methods for regenerating Physarum from its dormant sclerotia state, culturing plasmodia, and drying plasmodia to sclerotia. We describe methods for the modeling of injury and regeneration, particularly in complex environments like mazes and salt bridges. We also present methods for automated macrophotography to monitor Physarum growth and form. For more detailed analysis of Physarum form and function, we introduce microscopy approaches, including the injection of fluorescent polymers and beads prior to imaging. Those fluorescent materials can be used to observe the response of Physarum to localized nutrient or chemical stimuli via cytoplasmic shuttle streaming, which is the primary method of information transfer throughout the organism’s body [1]. Methods to quantify both the structural and functional Physarum networks during growth and repair are also described. Finally, we outline techniques to sample Physarum networks and plasmodial slime for downstream use in mass spectrometry metabolomics or gene expression measurements like polymerase chain reaction or RNA sequencing [11, 20].
2 Materials
Prepare all solutions using ultrapure water (0.2 μm-filtered and deionized). Prepare and store all reagents at room temperature (unless indicated otherwise).
2.1 Physarum Culture Environments
-
1.
Sample of Physarum as plasmodia or sclerotia (see Note 1).
-
2.
1% (w/v) agar plate medium: 3 g bacteriological agar powder (C12H18O9) in 300 mL water. Heat until complete dissolution (see Note 2).
- 3.
-
4.
Spray bottle of water.
-
5.
Humidity and temperature-controlled plant growth incubator (see Note 5).
-
6.
10% (w/v) flan medium: 10 g blended oat flakes in boiling 1% agar plate medium. Pour the mixture into molds to set and allow to acclimate to room temperature prior to use for experiments.
-
7.
Maze : 1 cm wide lanes cut out from 1 cm thick acrylic sheet. Fill lanes with a 0.25 cm thick layer 1% agar plate medium.
-
8.
Agar bridge: round food patch of 10% flan medium (diameter = 15 mm), round patch 1% agar (diameter = 15 mm). Inside a Petri dish, separate the patches by a rectangular platform composed of 1% agar gel, 1% agar gel with 150 mM NaCl, or other noxious substance (see Note 6). Keep agar bridges in individual Petri dishes for experiments. Adhere black paper to the inside of each Petri dish to absorb moisture in the dish, forcing the hydrophilic Physarum to traverse along the agar bridge, and providing clear contrast for imaging.
2.2 Physarum Imaging and Analysis
-
1.
Macro-photography setup (e.g. scanner, camera, or webcam): For continuous imaging, install the macro-photography setup inside of a dark, environmentally controlled chamber.
-
2.
Upright widefield microscope with brightfield & fluorescent channels.
-
3.
1× commercially available phosphate-buffered saline (PBS) (see Note 7).
-
4.
Non-toxic fluorescent polymer for visualization of shuttle streaming in plasmodial Physarum: Dilute to desired concentration using 1× PBS.
-
5.
Fluorescent beads: Dilute to desired concentration using 1× PBS.
-
6.
Glass capillary needle.
-
7.
Pressurized injector.
-
8.
Dissection microscope.
-
9.
Physarum Network Analysis Program (see Note 8).
-
10.
MATLAB, Octave, Scikit-Image or other high-level programming language with image analysis functions.
-
11.
Bioformats Package (Open Microscopy) for image import.
-
12.
Java (latest version).
-
13.
Ghostscript for exporting images in vector formats.
-
14.
Pdftops (Xpdf Suite; Foo Labs) for eps file export.
3 Methods
Carry out all procedures at room temperature unless otherwise specified.
3.1 Physarum Culture
-
1.
Pour 2 mL of sterile water onto a 1 cm2 filter paper containing dehydrated Physarum sclerotia (see Note 9).
-
2.
Place moistened filter paper, sclerotia-side down, at the center of a 1% agar-filled plate.
-
3.
Arrange 4–6 oatmeal flakes 3–5 cm from the sclerotia.
-
4.
Apply a single spray of sterile water on the plate to moisten, but not soak, the environment.
-
5.
Maintain cultures at 22 °C and 90% humidity in a dark incubator (see Note 5).
-
6.
Wait 2 days for the Physarum plasmodia to start growing toward the oat flake food sources (see Note 10).
-
7.
Add 10 to 12 oat flakes to cover the rest of the plate.
-
8.
Apply a single spray of sterile water to the plate.
-
9.
Return to incubator.
-
10.
Add fresh oat flakes every 2 days (see Note 10).
-
11.
Return to incubator.
-
12.
Wait a total of 5 days for Physarum to completely cover the plate.
-
13.
Cut a 1 cm2 cube of Physarum-coated agar using a sterile toothpick.
-
14.
Transfer the piece of agar, Physarum-up, to the center of a fresh 1% agar plate (Fig. 2a, b) to sub-culture the Physarum to a new plate.
-
15.
Spread 10 to 12 oat flakes across the agar.
-
16.
Apply a single spray with sterile water.
-
17.
Incubate at 22 °C and 90% humidity in the dark.
-
18.
Repeat steps 14–17 to maintain a growing culture of Physarum (see Note 11).
-
19.
Allow plasmodium to grow over filter paper to begin transition to the sclerotia state for long-term storage.
-
20.
Move the filter paper with plasmodium to dry, dark conditions for at least 2 days to produce sclerotia.
-
21.
Keep the sclerotia in dry and dark conditions for long-term storage. The dry sclerotia can remain dormant for up a year.
Physarum growth and repair. (a) Physarum grows from a single seed point (arrow) towards food sources (arrowheads) one day after sub-culturing. (b) After 3 days, the plasmodial Physarum covers the plate, forming a network that includes the seed point (arrow) and all food sources (arrowheads). (c) Before injury, a vein connects two food sources. The food source in the upper right corner is proximal to the Physarum seed point compared to the food source in the lower left corner. (d) An injury to the vein that leaves the underlying agar intact can be (e) rapidly repaired within 5 h
3.2 Models of Vein Repair After Injury & Amputation
The repair processes described here can be monitored using the imaging approaches outlined in Subheading 3.4.
-
1.
Take a growing Physarum from the culture incubator to be used for vein injury.
-
2.
Select a vein to be injured.
-
3.
Scrape the vein gently to create a 1 cm gap using a cell scraper without disrupting the underlying agar layer (Fig. 2c, d) (see Notes 12 and 13).
-
4.
Return the plate to its incubator.
-
5.
Image the plate hourly by automated, time-lapsed imaging using the macro-photography setup (Fig. 2e) as described in Subheading 3.4.
-
6.
Wait 5 h, or more, until the vein has repaired (see Note 13).
-
7.
Take a growing Physarum from the culture incubator to be used for vein amputation.
-
8.
Select a vein to be amputated.
-
9.
Scrap gently the whole vein to amputate it using a cell scraper without disrupting the underlying agar layer (see Notes 12 and 13).
-
10.
Image the plate hourly by time-lapsed imaging using the macro-photography setup (Fig. 2e).
-
11.
Repeat step 10 until the vein has repaired (see Note 13).
3.3 Generation in Complex Environments: Maze & Salt Bridge Experiments
The generative processes described here can be monitored using the imaging approaches outlined in Subheading 3.4.
-
1.
Take a growing Physarum from the culture incubator to be used for maze growth.
-
2.
Identify one oat flake that is evenly covered in Physarum.
-
3.
Place the Physarum-covered oat at the entrance of the maze.
-
4.
Add 2 fresh oat flakes at the maze’s endpoint and 3 at points equidistant from the start of the maze to induce growth away from the source (Fig. 3b).
-
5.
Move the maze to the incubator.
-
6.
Image the plate hourly by time-lapsed imaging using the macro-photography setup (Fig. 2e) as described in Subheading 3.4.
-
7.
Wait 48 h.
-
8.
Take a growing Physarum from the culture incubator to be used for a bridge experiment.
-
9.
Prepare one normal 1% agar bridge (control) and one 150 mM NaCl agar bridge.
-
10.
Place a Physarum sample on the 1% agar side of both agar bridges (Fig. 3c).
-
11.
Move plates to the incubator and allow 24 h for Physarum to traverse the bridge (Fig. 3d).
Physarum generation in complex environments. (a) Physarum grows within the confines of the maze (white arrows) toward the food source and retreats from areas lacking food (red arrow), building an optimized morphology based on the environmental conditions. (b) The oat flakes at the primary maze entrance points (blue arrows) are placed to encourage Physarum growth in three directions. Despite this, after 48 h Physarum only grows toward the maze endpoint (purple arrow; oatmeal flake removed). (c) The agar bridge connects Physarum samples to the 10% flan medium. Salt or other substances may be added to the bridge to induce morphological changes, such as (d) growth around the noxious salt bridge after 24 h
3.4 Structural & Functional Imaging
-
1.
Store the macro-photography set-up inside of a temperature and humidity-controlled incubator for automated time-lapse imaging over multiple days.
-
2.
Place the Physarum dish(es) in the field of view of the macro-photography set-up inside the incubator.
-
3.
Set the image acquisition rate and total imaging time to automatically acquire images (Fig. 4a) (see Note 14).
-
4.
Wait until the total imaging time has elapsed.
-
5.
Stop time-lapse imaging.
-
6.
Before brightfield and darkfield imaging, remove Petri dish lid to minimize reflection artifacts in images.
-
7.
Illuminate from above the microscope stage and place the Petri dish flat in the field of view.
-
8.
Use the brightfield view to visualize the outer architecture of the plasmodial network (Fig. 4b).
-
9.
To visualize the interior compartments of Physarum veins, use darkfield microscopy (Fig. 4c).
-
10.
Load fluorescent dextran or bead solution into glass capillary needle using a pressurized injection system for visualizing shuttle streaming within Physarum plasmodial networks.
-
11.
Place the tip of the glass capillary needle into a large, primary vein of the Physarum.
-
12.
Inject labeling solutions into the vein using short pulses (150 ms) at 140 kPa pressure.
-
13.
If necessary, clean and clear the capillary needle (see Notes 15 and 16).
-
14.
Repeat injections in other regions of the plasmodium, as needed.
-
15.
Visualize shuttle streaming using fluorescent microscopy in the same way described for brightfield and darkfield imaging (Fig. 4d, e; see Note 17).
-
16.
Set the microscopy software to acquire images at a high frame rate (typically ≥1 frame/s) to capture movement.
Structural and functional visualization of Physarum plasmodium. Physarum structure visualized using (a) a scanner, (b) brightfield microscopy, or (c) darkfield microscopy. Brightfield imaging captures the outer architecture of the Physarum, whereas darkfield imaging shows compartments within each vein (insets). (d) Fluorescent dextran travels through the Physarum network, with preference for certain paths (arrow) over others (star). Dextran can be shuttled through the network and deposited in slime secretions (box). (e) Fluorescent beads travel through the Physarum network (shown at high magnification)
3.5 Quantification of Growth & Repair
This section outlines a general protocol for Physarum image processing and network analysis.
-
1.
Launch the Physarum_network application (see Note 8).
-
2.
Import 8-bit images in the Directory panel (Fig. 5a).
-
3.
Input the microns per pixel in the Profile panel to calibrate the image size.
-
4.
Calculate the full width half maximum value for the smallest and largest veins in the network in the Profile panel to appropriately set the scaling and filtering operations for the network.
-
5.
Set the number of scales and resampling in the Image processing panel based on the full width half maximum values determined in the Profile panel.
-
6.
Select the opening function for background correction in the Image processing panel (see Note 18).
-
7.
Select the guided filtering algorithm in the Imaging processing panel to improve signal to noise in the image (see Note 18).
-
8.
Select process to visualize the filtered output in the image display panel (Fig. 5b).
-
9.
Select single projection in the network template panel to for converting a single original image to a network skeleton in the network template panel (Fig. 5c). For time series, a template can be constructed from a projection of the data along the time dimension (see Note 19).
-
10.
Leave the boundary mask options unchecked in the Skeleton extract panel (default) if there are no boundaries, regions to exclude, or food sources in the image. If there are non-Physarum objects in the image, use this panel to define boundaries, exclude regions, or delineate food sources (see Note 20).
-
11.
Use the Frangi kernel algorithm in the skeleton extract panel to improve the relative contrast of the network veins by enhancing vessel-like features through calculating the eigenvectors of the Hessian (Fig. 5d; see Note 21).
-
12.
Use the hysteresis algorithm to extract the skeleton network. This algorithm uses intensity information and pixel connectivity to derive a single-pixel skeleton.
-
13.
Check that the single-pixel skeleton accurately reflects the network in the original images (Fig. 5e).
-
14.
Manually edit the network skeleton if connections are incorrect or missing using the binary editing tool in the skeleton extract panel.
-
15.
Estimate vein diameter across the network using the distance approach, which estimates the local full-width half max from the original tubule intensity for each pixel in the skeleton.
-
16.
Convert the skeleton into a weighted graph representation of the Physarum network with nodes defined by the junctions between the veins and edges defined by each vein that joins nodes.
-
17.
Visualize graph edge metrics in the display panel or in the parameter panel (Fig. 5f).
-
18.
Output images and graph metrics for additional analysis.
Image processing and network analysis pipeline to quantify Physarum shape and connectivity. (a) Images saved from a scanner are imported and (b) filtered, (c) used to create a template, and (d) processed to enhance vein structure. The single pixel-wide skeleton is extracted and used to form a (e) network graph for evaluation using metrics, like (f) vein width. Scale bar = 1 cm for all panels
3.6 Sampling Physarum
-
1.
Remove oat flakes from the Physarum plate of interest.
-
2.
Using a cell scrapper, carefully remove the plasmodial network from the agar to sample the Physarum.
-
3.
Record the total mass of Physarum sample.
-
4.
Combine the sample with water or media of choice at a concentration of 1 g/mL.
-
5.
Vortex for 30 s to combine.
-
6.
Continue with sample centrifugation and storage in step 11.
-
7.
Remove oat flakes from an additional Physarum plate.
-
8.
Add water or other media to cover the plate in a thin layer (5 mL for a 140 mm plate) to sample water-soluble Physarum secretions.
-
9.
Mildly shake Physarum plates for 30 min to collect water-soluble components of the Physarum and its secretions.
-
10.
Holding the plate at an angle, collect liquid from the plate using a pipette.
-
11.
Centrifuge each sample at 3000 rcf for 15 min to separate large particulates.
-
12.
Collect the supernatant liquid from each centrifuged sample.
-
13.
Store samples at −80 °C for downstream analyses.
4 Notes
-
1.
Physarum culture kits can be purchased from several scientific retailers. We purchase sclerotia from Carolina Biological Supply Company.
-
2.
This recipe makes enough 1% agar medium for six 140 mm diameter dishes filled with 50 mL/dish.
-
3.
Physarum prefer nutrition from old fashioned or whole-oat oatmeal and will not grow as well using instant (processed) oatmeal flakes or irradiated oatmeal.
-
4.
An alternative to feeding Physarum with oatmeal flakes is to make or purchase oatmeal agar. The distribution of nutrition throughout the plate will cause Physarum to grow in dense clusters as opposed to networks. Shuttle streaming and the production of slime may be reduced using this method; however, oatmeal agar can be useful for culturing stock plates of Physarum.
-
5.
Physarum will grow most optimally if stored in a dark, humidity-controlled plant growth chamber. However, humid conditions can also be achieved in a standard incubator using a humidifier with automatic feedback control. Dry conditions can cause the plasmodial Physarum to revert to the dried, sclerotia state. Light can trigger spore formation instead of plasmodial growth [11].
-
6.
In addition to salt, other noxious substances can be integrated into the agar bridge, such as quinine and caffeine [9, 21].
-
7.
1× PBS is purchased in a formulation buffered to pH 7.4 and free of calcium chloride, magnesium chloride, and phenol red.
-
8.
The Physarum_network algorithm was developed by Fricker et al., 2017 and additional details about the algorithm are available [22].(Download website: https://markfricker.org/77-2/software/physarum-network-analysis/).
-
9.
Cultures can become contaminated, so inspect each plate for foreign cultures before subculturing or sampling for downstream analyses. To avoid contamination, use sterile instruments and keep Petri dishes covered when not actively working with them. Aliquot oatmeal flakes into 50 mL air-tight containers to prevent contamination of the food stocks.
-
10.
Some Physarum may grow faster. Once cultures cover all flakes, proceed to next steps. Because Physarum grow toward fresh food sources, add new flakes every 1–3 days for optimal growth.
-
11.
We recommend that Physarum undergo subculture weekly. Physarum can undergo continuous subculture cycles, however, the organism will exhibit signs of aging after extended subculture, such as slowing of growth within 162 days for some strains [23]. We initiate new cultures from sclerotia quarterly throughout the year to avoid any slowing of growth due to senescence.
-
12.
The induction of injury in Physarum can be done with any number of blunt tools. However, we do not recommend using very sharp instruments, like a scalpel, which may damage the underlying agar and prevent rapid repair of Physarum veins.
-
13.
We have observed faster and more robust repair mechanisms in newly plated Physarum (less than 48 h since sub-culture). Therefore, we recommend that vein injury and amputation be performed early after sub-culture.
-
14.
Exposure to light can alter the growth patterns of Physarum, such as photoavoidance and sporulation [24]. To reduce the effects of light exposure during imaging, we recommend increasing the scanning time interval and therefore acquiring images at the lowest allowable rate. We typically image Physarum plates for 1–3 days at intervals of 15 min or longer.
-
15.
Physarum lacks cell walls and their slime sheath is the primary layer of protection from injury and the environment. Positioning the needle in the slime sheath can cause it to clog and therefore we recommend frequent cleaning of the needle or delivering the dextran or bead by pipette on or near the Physarum veins.
-
16.
Alternatively, fluorescent dextran and bead solutions can also be delivered to the surface of the Physarum using a pipette. Deposit a 1–2 μL droplet of dextran or bead solution on the surface of a large, primary vein of the Physarum. The Physarum vein will absorb the droplet and distribute it throughout the plasmodium using shuttle streaming. This technique avoids the challenges of pressure injection, such as needle clogging. However, the injection technique provides greater specificity in delivery location.
-
17.
2 μm-diameter fluorescent beads (FluoSpheres carboxylate-modified microspheres, 2 μm; Life Technologies) and 10,000 molecular weight fluorescent dextran (Dextran, Rhodamine B, 10,000 MW, Neutral; Invitrogen) were used in the microscopy images shown in this chapter.
-
18.
When performing image processing, we used the opening function to correct the background and guided to improve signal-to-noise. The opening function uses a disk-shaped kernel to remove features smaller than the thinnest veins and estimates the local background surrounding each pixel in the Physarum network image. The background is subtracted from the original image. The guided algorithm applies an edge-preserving smoothing filter according to the intensities in the original image.
-
19.
Creating a template for a time series using the projection algorithm can be helpful since it will permit the construction of a network with consistent node and edge definitions across the whole image series.
-
20.
We recommend selecting manual to exclude boundaries (such as a plate edge) using drawing tools to outline the image bounds. Features (such as a food source) in the Physarum image can be selected using the manual tool and will be included as a node during network construction.
-
21.
When extracting the Physarum network skeleton, we found the neuriteness algorithm to produce excellent network images. However, this algorithm produced a network comprised of two parallel lines, forming the outline of each vein, instead of a single-pixel wide binary skeleton. Instead, we suggest using the Frangi enhancement algorithm.
References
Alim K, Andrew N, Pringle A, Brenner MP (2017) Mechanism of signal propagation in Physarum polycephalum. Proc Natl Acad Sci U S A 114:5136–5141. https://doi.org/10.1073/pnas.1618114114
Vallverdú J, Castro O, Mayne R et al (2018) Slime mould: the fundamental mechanisms of biological cognition. Biosystems 165:57–70. https://doi.org/10.1016/j.biosystems.2017.12.011
Whiting JGH, Jones J, Bull L et al (2016) Towards a Physarum learning chip. Sci Rep 6:1–14. https://doi.org/10.1038/srep19948
Murugan NJ, Kaltman DH, Jin H et al (2020) Mechanosensation mediates long-range spatial decision-making in an aneural organism. Adv Mater 33(34):e2008161. https://doi.org/10.1101/2020.03.20.985523
Iwayama K, Zhu L, Hirata Y et al (2016) Decision-making ability of Physarum polycephalum enhanced by its coordinated spatiotemporal oscillatory dynamics. Bioinspir Biomim 11:036001. https://doi.org/10.1088/1748-3190/11/3/036001
Reid CR, Latty T, Dussutour A, Beekman M (2012) Slime mold uses an externalized spatial “memory” to navigate in complex environments. Proc Natl Acad Sci U S A 109:17490–17494. https://doi.org/10.1073/pnas.1215037109
Tsuda S, Jones J (2011) The emergence of synchronization behavior in Physarum polycephalum and its particle approximation. Biosystems 103:331–341. https://doi.org/10.1016/j.biosystems.2010.11.001
Doerhoefer S, Khodyreva S, Safronov IV et al (1998) Molecular constituents of the replication apparatus in the plasmodium of Physarum polycephalum: identification by photoaffinity labelling. Microbiology 144:3181–3193. https://doi.org/10.1099/00221287-144-11-3181
Vogel D, Dussutour A (2018) Brainless learners. In: Adams DS, Levin M (eds) Ahead of the curve, vol 2. IOP Publishing, Bristol, pp 6-2–6-12
Dove W, Rusch H (2017) Growth and differentiation in Physarum Polycephalum. Princeton University Press, Princeton, New Jersey
Schaap P, Barrantes I, Minx P et al (2016) The physarum polycephalum genome reveals extensive use of prokaryotic two-component and metazoan-type tyrosine kinase signaling. Genome Biol Evol 8:109–125. https://doi.org/10.1093/gbe/evv237
Lobo D, Solano M, Bubenik GA, Levin M (2014) A linear-encoding model explains the variability of the target morphology in regeneration. J R Soc Interface 11:20130918. https://doi.org/10.1098/rsif.2013.0918
Levin M (2012) Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Biosystems 109:243–261. https://doi.org/10.1016/j.biosystems.2012.04.005
Jones J (2015) From pattern formation to material computation: multi-agent modelling of Physarum Polycephalum, vol 15. Springer, Berlin
Ikeuchi M, Ogawa Y, Iwase A, Sugimoto K (2016) Plant regeneration: cellular origins and molecular mechanisms. Dev 143:1442–1451. https://doi.org/10.1242/dev.134668
Takamatsu A, Takaba E, Takizawa G (2009) Environment-dependent morphology in plasmodium of true slime mold Physarum polycephalum and a network growth model. J Theor Biol 256:29–44. https://doi.org/10.1016/j.jtbi.2008.09.010
Zhu L, Kim SJ, Hara M, Aono M (2018) Remarkable problem-solving ability of unicellular amoeboid organism and its mechanism. R Soc Open Sci 5:180396. https://doi.org/10.1098/rsos.180396
Nakagaki T, Yamada H, Tóth A (2000) Maze-solving by an amoeboid organism. Nature 407:470
Bonifaci V, Mehlhorn K, Varma G (2012) Physarum can compute shortest paths. J Theor Biol 309:121–133. https://doi.org/10.1016/j.jtbi.2012.06.017
Golderer G, Werner ER, Leitner S et al (2001) Nitric oxide synthase is induced in sporulation of physarum polycephalum. Genes Dev 15:1299–1309. https://doi.org/10.1101/gad.890501
Boisseau RP, Vogel D, Dussutour A (2016) Habituation in non-neural organisms: evidence from slime moulds. Proc R Soc B Biol Sci 283:2–8. https://doi.org/10.1098/rspb.2016.0446
Fricker MD, Akita D, Heaton LLM et al (2017) Automated analysis of Physarum network structure and dynamics. J Phys D Appl Phys 50:1–15. https://doi.org/10.1088/1361-6463/aa72b9
Nakagawa CC, Jones EP, Miller DL (1998) Mitochondrial DNA rearrangements associated with mF plasmid integration and plasmodial longevity in Physarum polycephalum. Curr Genet 33:178–187. https://doi.org/10.1007/s002940050325
Nakagaki T, Umemura S, Kakiuchi Y, Ueda T (1996) Action Spectrum for sporulation and Photoavoidance in the plasmodium of Physarum polycephalum, as modified differentially by temperature and starvation. Photochem Photobiol 64:859–862
Acknowledgements
This research was also supported by the Allen Discovery Center program through The Paul G. Allen Frontiers Group (12171). We also gratefully acknowledge the Defense Advanced Research Projects Agency (DARPA) under Cooperative Agreement Number HR0011-18-2-0022. The content of the information does not necessarily reflect the position or the policy of the Government, and no official endorsement should be inferred. Approved for public release; distribution is unlimited. The authors also thank Melanie Chien for assistance with experiments and Cuong Nguyen for training in laser cutting during preparation of the maze.
Author information
Authors and Affiliations
Corresponding authors
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Sperry, M.M., Murugan, N.J., Levin, M. (2022). Studying Protista WBR and Repair Using Physarum polycephalum . In: Blanchoud, S., Galliot, B. (eds) Whole-Body Regeneration. Methods in Molecular Biology, vol 2450. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2172-1_3
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2172-1_3
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2171-4
Online ISBN: 978-1-0716-2172-1
eBook Packages: Springer Protocols