Abstract
B cell receptor (BcR) immunoglobulins (IG) display a tremendous diversity due to complex DNA rearrangements, the V(D)J recombination, further enhanced by the somatic hypermutation process. In chronic lymphocytic leukemia (CLL), the mutational load of the clonal BcR IG expressed by the leukemic cells constitutes an important prognostic and predictive biomarker. Here, we provide a reliable methodology capable of determining the mutational status of IG genes in CLL using high-throughput sequencing, starting from leukemic cell DNA or RNA.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
- Chronic lymphocytic leukemia
- Immunoglobulin genes
- Next generation sequencing
- Somatic hypermutation analysis
- Mutational status
1 Introduction
Chronic lymphocytic leukemia (CLL) is a malignant clonal proliferation of mature B cells. It is the most frequent leukemia in adults in the Western world and is characterized by a marked clinical heterogeneity. For some patients, it is an indolent disease with no or only late need of treatment, while in others it displays an aggressive behavior requiring early initiation of therapy [1]. Many prognostic factors have been identified, and among them, the mutational status of the immunoglobulin heavy chain variable (IGHV) genes of the B cell receptor (BcR) has emerged as one of the most robust parameters [2]. It has several advantages as it is stable and can be evaluated at any time including at diagnosis and is independent of other clinical or biological factors [3]. In addition, it has also proved to be a predictive factor of response to chemoimmunotherapy [4, 5]. Therefore the recent guidelines from the International Workshop on CLL recommend that determination of the IGHV mutational status should be performed before treatment initiation both in clinical trials and in general practice [6].
The BcR IG display huge diversity in their variable regions which results from complex mechanisms: (1) assembly of variable (V), diversity (D), and joining (J) genes, (2) imprecise junction of these rearranged genes with random nucleotide insertion and deletions, and (3) pairing of heavy and light chains [7]. Further diversification occurs after antigen encounter by somatic hypermutation in the V regions coupled with affinity maturation of the BcR [8]. In tumors such as CLL , all leukemic cells bear the same clonal BcR which reflects the developmental stage from which they derive and constitutes a biomarker of the disease.
Determination of IGHV mutational status is achieved by sequencing the IGHV gene from the clonal IGH rearrangement of the leukemic cells, followed by its comparison with the closest germline counterpart from which it derives [9]. An identity <98% classifies the CLL as “mutated” which is associated with a favorable outcome, while an identity ≥98% defines “unmutated” CLL and confers a poor prognosis [10, 11]. Since this initial observation in 1999, numerous studies have confirmed that unmutated CLL have shorter time-to-first treatment and overall survival when compared to mutated cases [2, 3]. In addition, large-scale repertoire analyses have shown that CLL display a skewed IG repertoire with a sizeable fraction of patients sharing quasi-identical IG variable heavy chain regions sequences, a phenomenon termed BcR IG stereotypy [12]. Importantly, some of these CLL cases belonging to the same stereotypic groups (or subsets) may also share similar clinical and biological features, separating them from other patients with the same IGHV mutational status [13, 14]. Therefore, BcR IG stereotypy further refines the categorization into mutated or unmutated CLL .
The European Research Initiative on CLL (ERIC) has published methodological guidelines and recommendations on how to perform and interpret IGHV mutational status in CLL [15]. The first step consists in polymerase chain reaction (PCR) amplification of clonal IGH rearrangements. Importantly, as the whole IGHV gene sequence is necessary for accurate calculation of the somatic hypermutation load, 5′ primers need to be positioned upstream, e.g., on the leader peptide. Both genomic DNA (gDNA) and RNA extracted from leukemic cells can serve as templates, with gDNA having the advantage of being a more robust material, simpler to obtain and also a source for other genomic investigations. However, in a fraction of cases, amplification from gDNA is hampered by the presence of somatic hypermutation in the primer binding sites. Although starting from RNA requires an additional step of reverse transcription (RT), this can be a useful alternative or complementary approach as it allows the use of primers binding to sequences less or not targeted by somatic hypermutation upstream and downstream of the IGHV-IGHD-IGHJ rearrangement, respectively, in the leader region L1 part and the constant regions.
Sequencing of the IGH rearrangements amplicons was traditionally performed by Sanger methodology. However, with the constant advance of next generation sequencing (NGS) in the diagnostic field, there is a need to adapt this technology to IGHV mutational determination [16, 17]. Here, we describe detailed protocols for NGS-based determination of the IGHV mutational in CLL , starting from either gDNA or cDNA templates.
2 Materials
2.1 Sample Preparation
-
1.
50 mL polypropylene tubes.
-
2.
UNI-SEPMaxi (EUROBIO Scientific).
-
3.
Phosphate-buffered saline (1×PBS) pH 7.4 (Thermo Fisher Scientific).
-
4.
Centrifuge.
-
5.
1.5 mL microfuge tubes.
-
6.
Blood and Cell Culture DNA kit (Qiagen).
-
7.
RNAeasy Mini Kit (Qiagen).
-
8.
Nanodrop ND1000 (Thermo Fisher Scientific).
-
9.
Nuclease-free water (Promega).
-
10.
Thermocycler.
2.2 Primer Preparation
-
1.
Primers (Eurogentec).
-
2.
Nuclease-free water (Promega).
-
3.
1.5 mL microfuge tubes (Eppendorf).
2.3 PCR Amplification
- 1.
-
2.
High Fidelity Platinum® Taq DNA Polymerase, 5 U/μL (with 10× High Fidelity PCR Buffer and 50 mM MgSO4) (Thermo Fisher Scientific).
-
3.
dNTP Mix, 10 mM (Thermo Fisher Scientific).
-
4.
Nuclease-free water (Promega).
-
5.
0.2 mL 96-well PCR plate (AB-0600) (Thermo Fisher Scientific).
-
6.
Adhesive sealing sheets (Dominique Dutscher).
-
7.
Thermocycler (typically: Applied Biosystems Veriti 96).
2.4 PCR Product Purification
-
1.
Agencourt AMPure XP beads (Beckman Coulter).
-
2.
Ethanol absolute (VWR).
-
3.
Magnetic Stand-96 (Invitrogen).
-
4.
Nuclease-free water (Promega).
-
5.
0.2 mL 96-well PCR plate (AB-0600) (Thermo Fisher Scientific).
-
6.
Microplate shaker (Eppendorf).
-
7.
Microplate centrifuge.
2.5 Quantification of Purified PCR Products
-
1.
Quant-iT™ dsDNA High-Sensitivity Assay Kit (Thermo Fischer Scientific).
-
2.
Microplate fluorescence reader, such as Clariostar (BMG Labtech).
-
3.
MicroPlate 96-well, F-bottom (chimney well), black (Greiner).
-
4.
Adhesive PCR sealing foil sheets (Thermo Fisher Scientific).
-
5.
Microplate shaker (Eppendorf).
-
6.
Microplate centrifuge.
2.6 Library Preparation
-
1.
0.2 mL 96-well PCR plate (AB-0600) (Thermo Fisher Scientific).
-
2.
1.5 mL microfuge tubes (Eppendorf).
2.7 Library Denaturation and Illumina MiSeq Sequencing
-
1.
Sodium hydroxide (NaOH) 1 N (VWR).
-
2.
Nuclease-free water (Promega).
-
3.
1.5 mL microfuge tubes (Eppendorf).
-
4.
EBT: 10 mM Tris-Cl, pH 8.5 (Buffer EB Qiagen) with 0.1% Tween 20 (Euromedex).
-
5.
PhiX control (10 nM) (Illumina).
-
6.
MiSeq Reagent Kit v3 (600 cycles) (Illumina) including cartridge, HT1 buffer, flow cell and sequencing buffer.
-
7.
MiSeq System (Illumina).
2.8 Bioinformatics Analysis
-
1.
Vidjil platform account (support@vidjil.org).
3 Methods
3.1 Template Preparation (See Note 1)
3.1.1 Lymphocyte Isolation from Peripheral Blood Using Density Gradient Separation
-
1.
Slowly add 10–17.5 mL of blood to a UNI-SEP Maxi tube.
-
2.
Centrifuge at 1000 × g for 15 min.
-
3.
Collect the mononuclear cell ring above the membrane and transfer to a 50 mL tube; fill up to 50 mL with PBS 1×.
-
4.
Centrifuge at 600 × g for 10 min, and then discard the supernatant.
-
5.
Resuspend the cell pellet in 1 mL of PBS and then fill the tube with PBS 1×.
-
6.
Centrifuge at 600 × g for 10 min, and then discard the supernatant.
-
7.
Repeat steps 5 and 6 of this section.
-
8.
Transfer the cell pellet into a 1.5 mL microfuge tube and remove all remaining supernatants.
3.1.2 Genomic DNA Extraction
-
1.
Extract gDNA from cell pellets or tissue biopsy with the Qiagen DNA kit following the manufacturer’s instructions.
-
2.
Quantify DNA by spectrophotometry (Nanodrop) and adjust to a final working concentration of 20 ng/mL with nuclease-free water.
3.1.3 RNA Extraction and cDNA Synthesis
-
1.
Extract RNA with the Qiagen RNAeasy Mini kit according the manufacturer’s instructions and then quantify on a Nanodrop spectrophotometer.
-
2.
Dilute 1 μg of RNA in a microcentrifuge tube with nuclease-free water in a 10 μL total volume and incubate 10 min at 70 °C.
-
3.
Add 10 μL of the RT mix containing: 2 μL RT buffer (10×), 0.8 μL dNTP mix (25×), 2 μL random primers (10×), 1 μL MultiScribe reverse transcriptase (50 U/μL), 1 μL RNAase inhibitor (20 U/μL), 3.2 μL nuclease-free water.
-
4.
Place in a thermocycler with the following program: 10 min at 25 °C, 120 min at 37 °C, 5 min at 85 °C, 4 °C on hold.
-
5.
Add 30 μL nuclease-free water.
-
6.
At this stage the cDNA mixture can be stored at −80 °C (see Note 2).
3.2 Primer Preparation
3.2.1 Primer Preparation for gDNA Template
Primer sequences are indicated in Table 1 (see Notes 3 and 4).
-
1.
Prepare a 100 μM forward primer mix by pooling each of the 24 IGHV-Leader L2-part primers, all bearing the same barcode, in a microcentrifuge tube. Further dilute this primer mix with nuclease-free water to a final 20 μM concentration.
-
2.
Dilute the reverse IGHJ primer with nuclease-free water to a final 5 μM concentration (see Note 5).
3.2.2 Primer Preparation for cDNA Template
Primer sequences are indicated in Table 2 (see Notes 3 and 4).
-
1.
Prepare a 100 μM forward primer mix by pooling each of the 6 IGHV-Leader L1-part primers, all bearing the same barcode, in a microcentrifuge tube.
-
2.
Prepare a 100 μM reverse primer mix by pooling each of the 2 IGHC primers, all bearing the same barcode, in a microcentrifuge tube. Further dilute these primer mixes with nuclease-free water to a final 5 μM concentration (see Note 5).
3.3 PCR Amplification of IGH Rearrangements
-
1.
Thaw, mix, and briefly centrifuge each component before use.
-
2.
Prepare a PCR master mix by adding the components as shown in Table 3 for gDNA or Table 4 for cDNA.
-
3.
Dispense 40 μL of this mix in each well of the plate.
-
4.
Add 3 μL of forward primer mix.
-
5.
Add 2 μL of reverse primer mix.
-
6.
Add 5 μL of gDNA or cDNA template.
-
7.
Seal the plate with adhesive sheet.
-
8.
Shake briefly and centrifuge (short pulse).
-
9.
Place the plate in a thermocycler. The PCR program is the following: denaturation at 95 °C for 3 min; 35 cycles of 95 °C for 45 s, 63 °C for 45 s, 68 °C for 1 min; final extension at 68 °C for 10 min; 12 °C on hold.
-
10.
At this stage, the plate can be sealed and stored at −20 °C for later usage.
3.4 PCR Product Purification
-
1.
Preparation.
-
(a)
Prepare fresh 70% ethanol for optimal results.
-
(b)
Agencourt AMPure XP bottle should be used at room temperature.
-
(a)
-
2.
Centrifuge briefly the PCR plate.
-
3.
Shake the Agencourt AMPure XP bottle to resuspend the magnetic beads before adding 37.5 μL per well of the PCR plate (see Note 6).
-
4.
Mix thoroughly by pipetting until the mixture appears homogeneous.
-
5.
Incubate for 5 min at room temperature.
-
6.
Place the reaction plate on the magnetic stand for 5 min.
-
7.
Remove and discard the cleared supernatant (80 μL).
-
8.
Wash the beads by dispensing 200 μL of 70% ethanol (freshly prepared) to each well of the reaction plate, and incubate for 30 s at room temperature; then aspirate and discard the ethanol (200 μL).
-
9.
Repeat for a total of two washes.
-
10.
Dry 5 min at room temperature to ensure all traces of ethanol are removed.
-
11.
To elute purified DNA fragments from beads, remove the reaction plate from the magnetic stand, and then add 35 μL of nuclease-free water to each well of the reaction plate and mix by pipetting until beads are completely resuspended.
-
12.
Incubate for 5 min at room temperature.
-
13.
Place the reaction plate onto the magnetic plate for 2 min to collect the beads.
-
14.
Transfer 25 μL of the eluate to a new microplate.
-
15.
At this stage, the plate can be sealed and stored at −20 °C for later usage.
3.5 Quantification of Purified PCR Products (See Note 7)
-
1.
Dilute Quant-iT™ dsDNA HS reagent 1:200 in Quant-iT™dsDNA HS buffer (sufficient quantity for all PCR samples plus 8 standards and 1 blank).
-
2.
Load 200 μL of the working solution into each microplate well.
-
3.
Add 10 μL of each dsDNA HS standards or 2.5 μL of each PCR sample.
-
4.
Place on the plate shaker at 1200 rpm for 5 min.
-
5.
Briefly spin in a centrifuge.
-
6.
Measure the fluorescence using the Clariostar microplate reader.
-
7.
Use the standard curve to determine the DNA amounts (see Note 8).
3.6 Library Preparation and Quantification
-
1.
Calculate the concentration in nM according to the formula:
$$ \left[\mathrm{concentration}\left(\mathrm{ng}/\upmu \mathrm{L}\right)/\right(\mathrm{size}\ \mathrm{amplicon}\left(\approx 550\;\mathrm{bp}\right)\times 650\Big]\times {10}^6. $$ -
2.
For samples with purified PCR products >10 nM:
-
(a)
Dilute samples in a new microplate to final 10 nM concentration (5 μL PCR product + H2O up to 10 nM using the formula: vol. H2O = [conc(nM)/2] − 5.
-
(b)
Use 5 μL of this 10 nM dilution for pooling samples in a microfuge tube.
-
(a)
-
3.
For samples with purified PCR products <10 nmm, use 10 μL for library pooling in the same microfuge tube.
-
4.
At this stage, the tube containing the library pool can be sealed and stored at −20 °C for later use.
3.7 Library Denaturation and Illumina MiSeq Sequencing
-
1.
Place the MiSeq Reagent Kit at 4 °C the day before to thaw the reagents overnight.
-
2.
On the day of the run, prepare 1 mL of NaOH 0.2 N in a microcentrifuge tube: 200 μL 1 N NaOH +800 μL H2O.
The following steps (steps 3–9) should be performed on ice:
-
3.
Dilute library at 4 nM in a microcentrifuge tube: add 2 μL library at 10 nM and 3 μL EBT.
-
4.
Denature and dilute library at 2 nM by adding 5 μL of 0.2 N NaOH.
-
5.
Incubate for 5 min at room temperature.
-
6.
Add 990 μL of HT1 buffer resulting in 1 mL of a 20 pM denatured library.
-
7.
Proceed the same way (i.e., steps 3–6) to obtain 20 pM denatured PhiX.
-
8.
In a new microcentrifuge tube, add 300 μL of 20 pM library to 300 μL HT1 resulting in 600 μL of 10 pM library; mix by pipetting.
-
9.
In a new microcentrifuge tube, add 540 μL of the 10 pM library and 60 μL of 20 pM denatured PhiX; mix by pipetting.
-
10.
Load 600 μL of the final combined library in the “load sample well” of the MiSeq cartridge.
-
11.
Enter the following parameters in the Local Run Manager (LRM) of the Miseq as depicted in Table 5.
-
12.
Fill the sample table with sample ID, and the associated index well (A01 corresponding to the unique indexes D701 and D501 combination).
3.8 Bioinformatic Analysis on Vidjil Platform (See Note 9)
-
1.
After completion of the run (56 h), copy the FASTQ files from the MiSeq directory on a hard drive.
-
2.
Connect to your Vidjil server and enter login and password (see Note 10).
-
3.
Create a run: click on run, and then click on [+ new runs]; enter run ID, run name, date, and other information, and click on [save].
-
4.
Create new patients for each sample in the run: click on [+ new patients] and enter patient ID, first name, last name, and other information; click on [save].
-
5.
Open the created run and click on [+ add samples].
-
6.
Choose pre-process scenario (read merging with Flash2): select [4- M + R2: Merge paired-end reads].
-
7.
Sample1: select R1 (first file) and R2 (second file) FASTQ files by clicking on [Browse…]. Date of sampling and other informations can be added. Importantly, the corresponding patient had to be associated with the sample by clicking its ID in the field [sample information].
-
8.
Repeat for each sample [add other sample].
-
9.
Click on [submit samples].
-
10.
In the created run select process config [IGH ] and click on the gear wheel to launch the analysis. The results are available when Completed appears in the status.
-
11.
To access results, go to the patient page and click on [IGH ].
-
12.
The following information is displayed: (see also Fig. 1).
-
(a)
List of most abundant clonotypes on the left.
-
(b)
Graphic visualization by abundance and read length (on top).
-
(c)
Graphic visualization by abundance and V/J usage (on the bottom).
-
(a)
-
13.
Select the most abundant clonotype (s).
-
14.
Click on the curved arrow on the bottom of the page to send the clonotype sequence to IMGT/V-QUEST [18] and ARResT/AssignSubsets [19] (see Note 11).
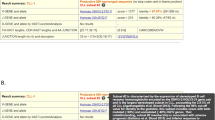
Screenshot of results displayed Vidjil. The Vidjil platform provides an interactive visualization of antigen receptor repertoire from high-throughput data. The left panel lists the most frequent clonotypes, the most abundant being at the top (squared). By default, the 50 most frequent ones are displayed, the value being adjustable (from 5 to 100). The IGHV, IGHD, and IGHJ genes contributing to each clonotype are indicated as well as number of deleted/inserted nucleotides. Further information is available by clicking on the yellow triangles. At the top of the left panel, a summary of the sample sequencing quality data can be obtained by clicking on the “i” symbol. The top-right panel shows the size distribution of the clonotype average read length, simulating the traditional Genescan view of clonality analysis. The bottom-right panel offers a representation of the clonotypes according to their size and IGHV and IGHJ gene composition. Note that, in the vast majority of cases, the CLL dominant IGH clonotype appears surrounded by multiple small variant ones, differing by minor nucleotides changes. Sequence of the selected clonotype appears at the very bottom, the IGHV, IGHD, and IGHJ genes being highlighted. By clicking on the bent arrow above (circled), the sequence is sent automatically to IMGT/V-QUEST, IgBlast, and ARResT/AssignSubsets for further analysis
4 Notes
-
1.
Peripheral blood is the most common source of material and should be collected (10–20 mL) in EDTA (or citrate)-containing tubes. Tumor material can also be obtained from tissues infiltrated by leukemic cells such as bone marrow or lymph nodes. Frozen biopsies are much preferred over formalin-fixed paraffin-embedded tissue samples due to the need to amplify relatively large PCR products (median size around 400 bp).
-
2.
If necessary, the quality of the cDNA synthesis can be assessed by amplification of a house-keeping gene, although this is not a mandatory step.
-
3.
The sequencing protocol uses dual index PCR primers. Each primer contains, from 5′ to 3′, the following: (1) a set of nucleotides for flow cell binding (P5 or P7), (2) a patient barcode index (forward D501-D508, reverse D701-D712), (3) a set of nucleotides for sequencing initiation, and (4) IGHV-Leader or IGHJ (or IGHC) specific primer sequence. The double barcode indexing allows up to 96 unique combinations in a single run.
-
4.
Primers are ordered according to a standard quality synthesis followed by polyacrylamide gel electrophoresis purification. They are resuspended in nuclease-free water at a 100 μM concentration.
-
5.
When preparing primer mixes, only IGHV-Leader and IGHJ or IGHC primers containing the same barcode can be pooled. Take precautions to avoid cross-contamination between primers with different barcodes.
-
6.
This 0.75:1 (beads/PCR products) ratio allows selective recovery of DNA fragments above 150 bp, thus eliminating primer dimers.
-
7.
Quantification of purified PCR products should be done preferentially by fluorometry. Several types of fluorescence readers can be used, including Qubit® 4 Fluorometer (Thermo Fischer Scientific) or Clariostar (BMG Labtech). Only the latter is described here.
-
8.
In case of concentration above the standards (upper limit 40 ng/μL), dilute the sample and repeat quantification.
-
9.
There are numerous tools to analyze antigen receptor sequences produced by high-throughput sequencing [20]. Here we refer to Vidjil (https://app.vidjil.org/) [21, 22], an easy-to-use platform which does not require specific informatics skills. Note that the current online version is for research only, but an option compliant for clinical use can be purchased. ArresT/Interrogate developed within the EuroClonality-NGS working group is another well-adapted alternative [23].
-
10.
Several options exist for adding patient data on a Vidjil server, see http://www.vidjil.org/doc/healthcare/
-
11.
More detailed information can be found in the user manual: http://www.vidjil.org/doc/user.
References
Hallek M, Shanafelt TD, Eichhorst B (2018) Chronic lymphocytic leukaemia. Lancet 391:1524–1537
Chiorazzi N, Stevenson FK (2020) Celebrating 20 years of IGHV mutation analysis in CLL. Hemasphere 4:e334
Sutton LA, Hadzidimitriou A, Baliakas P et al (2017) Immunoglobulin genes in chronic lymphocytic leukemia: key to understanding the disease and improving risk stratification. Haematologica 102:968–971
Rossi D, Terzi-di-Bergamo L, De Paoli L et al (2015) Molecular prediction of durable remission after first-line fludarabine-cyclophosphamide-rituximab in chronic lymphocytic leukemia. Blood 126:1921–1924
Fischer K, Bahlo J, Fink AM et al (2016) Long-term remissions after FCR chemoimmunotherapy in previously untreated patients with CLL: updated results of the CLL8 trial. Blood 127:208–215
Hallek M, Cheson BD, Catovsky D et al (2018) iwCLL guidelines for diagnosis, indications for treatment, response assessment, and supportive management of CLL. Blood 131:2745–2760
Tonegawa S (1983) Somatic generation of antibody diversity. Nature 302:575–581
Rajewsky K (1996) Clonal selection and learning in the antibody system. Nature 381:751–758
Lefranc MP, Giudicelli V, Duroux P et al (2015) IMGT®, the international imMunoGeneTics information system® 25 years on. Nucleic Acids Res 43:D413–D422
Damle RN, Wasil T, Fais F et al (1999) Ig V gene mutation status and CD38 expression as novel prognostic indicators in chronic lymphocytic leukemia. Blood 94:1840–1847
Hamblin TJ, Davis Z, Gardiner A et al (1999) Unmutated Ig V(H) genes are associated with a more aggressive form of chronic lymphocytic leukemia. Blood 94:1848–1854
Stamatopoulos K, Agathangelidis A, Rosenquist R et al (2017) Antigen receptor stereotypy in chronic lymphocytic leukemia. Leukemia 31:282–291
Baliakas P, Hadzidimitriou A, Sutton LA et al (2014) Clinical effect of stereotyped B-cell receptor immunoglobulins in chronic lymphocytic leukaemia: a retrospective multicentre study. Lancet Haematol 1:e74–e84
Sutton LA, Young E, Baliakas P et al (2016) Different spectra of recurrent gene mutations in subsets of chronic lymphocytic leukemia harboring stereotyped B-cell receptors. Haematologica 101:959–967
Rosenquist R, Ghia P, Hadzidimitriou A et al (2017) Immunoglobulin gene sequence analysis in chronic lymphocytic leukemia: updated ERIC recommendations. Leukemia 31:1477–1481
Langerak AW, Brüggemann M, Davi F et al (2017) High-throughput immunogenetics for clinical and research applications in immunohematology: potential and challenges. J Immunol 198:3765–3774
Davi F, Langerak AW, de Septenville AL et al (2020) Immunoglobulin gene analysis in chronic lymphocytic leukemia in the era of next generation sequencing. Leukemia 34:2545–2551
Brochet X, Lefranc MP, Giudicelli V (2008) IMGT/V-QUEST: the highly customized and integrated system for IG and TR standardized V-J and V-D-J sequence analysis. Nucleic Acids Res 36:W503–W508
Bystry V, Agathangelidis A, Bikos V et al (2015) ARResT/AssignSubsets: a novel application for robust subclassification of chronic lymphocytic leukemia based on B cell receptor IG stereotypy. Bioinformatics 31:3844–3846
Chaudhary N, Wesemann DR (2018) Analyzing immunoglobulin repertoires. Front Immunol 9:462
Giraud M, Salson M, Duez M et al (2014) Fast multiclonal clusterization of V(D)J recombinations from high-throughput sequencing. BMC Genomics 15:409
Duez M, Giraud M, Herbert R et al (2016) Vidjil: a web platform for analysis of high-throughput repertoire sequencing. PLoS One 11:e0166126. [Erratum in: PLoS One (2017) 12:e0172249]
Bystry V, Reigl T, Krejci A et al (2017) ARResT/Interrogate: an interactive immunoprofiler for IG/TR NGS data. Bioinformatics 33:435–437
Acknowledgments
Anne Langlois de Septenville and Myriam Boudjoghra contributed equally to this work.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Langlois de Septenville, A. et al. (2022). Immunoglobulin Gene Mutational Status Assessment by Next Generation Sequencing in Chronic Lymphocytic Leukemia. In: Langerak, A.W. (eds) Immunogenetics. Methods in Molecular Biology, vol 2453. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2115-8_10
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2115-8_10
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2114-1
Online ISBN: 978-1-0716-2115-8
eBook Packages: Springer Protocols