Abstract
During the last decade, therapeutic oligonucleotide drugs (OND) have witnessed a tremendous development in chemistry and mechanistic understanding that have translated into successful clinical applications. Depending on the specific OND mechanism, chemistry, and design, the DMPK and toxicity properties can vary significantly between different OND classes and delivery approaches, the latter including lipid formulations or conjugation approaches to enhance productive OND uptake. At the same time, with the only difference between compounds being the nucleobase sequence, ONDs with same mechanism of action, chemistry, and design show relatively consistent behavior, allowing certain extrapolations between compounds within an OND class. This chapter provides a summary of the most common toxicities, the improved mechanistic understanding and the safety assessment activities performed for therapeutic oligonucleotides during the drug discovery and development process. Several of the considerations described for therapeutic applications should also be of value for the scientists mainly using oligonucleotides as research tools to explore various biological processes.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Keywords
- Oligonucleotide drugs
- ASO
- siRNA
- Antisense
- Non-clinical safety assessment
- Preclinical safety assessment
- Toxicity
1 Introduction: Oligo Classes, Chemistries, and Designs
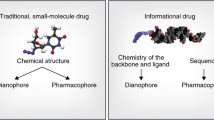
Oligonucleotide drugs (OND) of different classes range from 10–12 up to 100 nucleotides in length, often with chemical modifications of the backbone and ribose sugar. The chemistry and design used are dictated by the desired mechanism of action, resulting in different classes of therapeutic oligos with specific properties. The most common classes in clinical studies target RNA and rely on Watson-Crick hybridization for selectivity and affinity, where antisense oligonucleotides (ASOs) and short interfering RNAs (siRNAs) are the most common, with several examples of approved products [1, 2].
ASOs are single-stranded ONDs of 12–20 nucleotides in length, often with a phosphorothioate (PS) backbone and 2′ribose modifications like OMe, MOE, LNA, and cEt that improve drug properties like metabolic stability, tissue uptake, and increased affinity to the target transcript [3,4,5]. The ASO class can be further subdivided into ASO gapmers that trigger RNase H-mediated cleavage and degradation of target transcripts and steric blocking ASOs that modulate splicing events or inhibit activity of, e.g., microRNA (miR) [6,7,8]. For ASOs with a steric blocking activity, use of other chemistries resulting in neutral backbones like Phosphorodiamidate Morpholinos (PMO), Peptide nucleic acids (PNA) and tricyclo-DNA (tcDNA) is possible and quite common [9,10,11,12,13].
siRNAs and microRNA-mimics have a double-stranded design with each strand approximately 20–24 nucleotides in length and rely on loading of the antisense strand into RISC for activity [14, 15]. The design of siRNA results in cleavage and subsequent degradation of the target transcript , whereas miR mimics regulate gene expression by binding to miR sites in mRNA , inducing degradation and regulating protein translation [8].
In addition to hybridization dependent ASOs, miR mimics, and siRNA , there are several classes of hybridization independent ONDs including aptamers [16, 17] and immunostimulatory CpG oligos [18,19,20,21]. Rather than binding to RNA, the three-dimensional structure of folded RNA of a given sequence is combined with chemical modifications to achieve specific binding to proteins. Other therapeutic approaches such as mRNA therapy and the guide RNA in various gene editing approaches (e.g., CRISPR/CAS9) utilize nucleotides and but are commonly not classified as ONDs.
This chapter will focus on safety assessment of the hybridization dependent PS backbone ASOs and siRNA .
2 Delivery
The activity of ASOs, siRNA , and miR mimics rely on reaching the interior of the target cells. Uptake per se is not enough; the oligo needs to access the right subcellular compartments for activity, e.g., the cytosol for RISC loading or the nucleus for splice modulation or RNase H activity. Cellular uptake leading to pharmacodynamic effects are often referred to as “productive uptake” [22,23,24]. For ASOs, the best productive uptake after systemic delivery is often observed in the liver, in particular hepatocytes [24, 25]. Early siRNA candidates were delivered in protective formulations that also showed best uptake into hepatocytes [26]. Local delivery has successfully been used to bypass the blood–brain barrier, resulting in good therapeutic effects after local delivery to CNS [27,28,29], and the eye [30,31,32,33]. However, for use of therapeutic oligos beyond hepatocytes [3, 5, 34,35,36,37] and local delivery, achieving sufficient productive uptake has become one of the biggest challenges and numerous ways to overcome this has been proposed [36, 38, 39]. One approach is delivery in different types of formulations like the lipid nanoparticle (LNP) used to deliver Onpattro [26], the first siRNA receiving regulatory approval by FDA in 2018 [40]. However, although efficient delivery at low doses can be achieved, the LNP triggers proinflammatory flu-like responses that are managed by pre-medication before administration [41]. Since discovery of this LNP, a number of alternative formulations have been presented with different tissue distribution and improved efficacy:safety relationship.
An alternative way to improve the productive uptake is conjugation of the oligo to a targeting ligand, utilizing binding to cell surface receptors that internalize the oligo conjugate. An excellent example of this strategy is conjugation of the GalNAc carbohydrate, resulting in significantly improvements in productive uptake of both siRNA and ASOs [42, 43]. This GalNAc-mediated improvement in productive uptake is mediated by the binding of the conjugate to the asialoglycoprotein receptor (ASGR), which mainly is expressed on hepatocytes. Although non-conjugated ASOs show hepatocyte activity, adding GalNAc conjugates increased the clinical potency 20–30-fold for several re-formatted ASOs with hepatocyte targets [44]. Combining GalNAc conjugation with nuclease-resisting chemical stabilization has led to a tremendous increase in the utility of siRNA in the clinic, with several recent approvals with no need for the pre-medication required for LNP formulated Onpattro [2].
3 Safety Assessment of Therapeutic Oligos
The focus of this chapter is the preclinical safety assessment of therapeutic oligos intended to enter clinical trials to get regulatory approval for use in patients. With increasing experience and mechanistic understanding, screening cascades, study designs and data interpretations for ONDs are constantly improving, leading to more potent clinical candidates with better safety profiles. As described below, safety assessment of clinical OND candidates is a highly regulated process that at first sight could be of less interest for scientists primarily using oligonucleotides as tools to dissect and understand basic biological process. However, several of the considerations for bringing safe candidates to clinical trials could also be of value when developing optimal tool oligos and study designs for basic research. This includes cross species activity, restricted uptake distribution, long tissue half-life and effect duration, hybridization dependent off-target effects and the need to screen away from sequence dependent toxicities.
3.1 Discovery Phase: Selecting the Oligo Candidate with the Best Balance Between Potency and Safety
The potential safety concerns with therapeutic oligos can be divided into:
-
1.
Sequence and hybridization dependent (Sect. 3.1.1).
-
2.
Sequence and hybridization independent (Sect. 3.1.2).
-
3.
Sequence dependent, but hybridization independent (Sect. 3.1.3).
3.1.1 Sequence and Hybridization Dependent Effects: Assessing On- and Off-Target Safety
On-target toxicities, also known as exaggerated pharmacology, are dependent on sequence and RNA hybridization for ASOs, siRNA , and miR mimics. It can manifest in too strong intended effect or an adverse consequence of the pharmacological response in an unintended organ. A first assessment of potential on-target safety risks should be a theoretical exercise compiling available information on the biological role, tissue expression pattern, competitor information, etc. to assess the likelihood and potential adverse impact of the identified risks in the intended patient population. Considerations for assessing on-target toxicities for ONDs has been discussed by Kornbrust et al. [45].
Another safety concern dependent on Watson-Crick base pairing is hybridization dependent off-target risks. In contrast to the risk for on-target toxicity described above where the oligo has the intended, but exaggerated activity, this off-target risk relates to oligo activity on other transcripts than the intended target. Key features determining likelihood for hybridization dependent off-target effects have been discussed in depth elsewhere [46,47,48,49,50] and several recommendations [51] are summarized below:
-
1.
Identify candidates for off-target hybridization by in silico screening of the entire transcriptome of the pre-mRNA.
-
2.
In vitro confirmation: experimentally assess potency of sequences meeting the in silico criteria in vitro, establishing margins to activity on the primary target transcript .
-
3.
For off-targets with insufficient in vitro margins, assess potential consequences using the principles for on-target safety assessment.
There are some specific considerations when assessing potential hybridization-dependent safety concerns. First, due to species differences in sequence, it is often difficult to achieve pharmacological activity with the same OND in other species and it is common practice to use surrogate molecules with sufficient potency in the model species of choice.
Second, the chemical modifications used in therapeutic oligos lead to slow tissue elimination and extended effect duration [52]. This is convenient from a delivery perspective, but the washout period needed for the adverse effects to cease would be equally long should on- or off-target toxicities be observed.
Third, the restricted productive uptake of ASOs and siRNA to many cell types needs to be considered, as there is a large difference in the uptake between different cells and tissues. The oligo distribution can change with other administration routes and delivery systems like conjugates or formulations, so understanding the productive uptake distribution for such new conditions is critical for proper risk assessment of potential on- and off- target toxicities.
3.1.2 Sequence and Hybridization Independent Effects: Coagulation Time and Complement Activation
A couple of toxicities that are dependent on plasma Cmax but independent of both hybridization and sequence can be observed at relative high doses of PS backbone ASOs. This includes prolongation of coagulation time and activation of the alternative complement system. Acutely, activation of the alternative complement system can lead to significant drops in blood pressure. Repeated complement activation can result in “consumption” of complement factor C3 with impaired complement-mediated clearance of antibody aggregates resulting in vascular inflammation [53]. Data from in vitro, in vivo, and clinical studies clearly show that cynomolgus monkeys are significantly more sensitive than humans for this lowered threshold of complement activation [54, 55].
Both these effects are driven by the plasma Cmax levels [56,57,58,59,60] and transient in nature. With increased potency of modern ASOs and adapted dosing in current clinical studies, plasma concentrations rarely exceed these activation thresholds [55, 61, 62].
3.1.3 Sequence Dependent, But Hybridization Independent: Inflammation, Liver, and Kidney Toxicities
In contrast to these plasma Cmax-driven effects, other hybridization independent toxicities are highly dependent on the OND sequence. This includes proinflammatory manifestations and effects in high exposure organs such as liver and kidney that can sometimes be observed during the discovery phase. For siRNA , liver toxicity has been explained to be caused by off-target effects in the seed-region of the siRNA [48]. For PS backbone ASOs, liver toxicity is more frequently observed with higher affinity chemistry like LNA and cEt. It is clear from a number of published and unpublished observations that this liver toxicity observed during the screening process of PS backbone ASO gapmers is not caused by knockdown of the intended target transcript or liver concentration per se, see e.g. [63]. ASO sequence motifs associated with liver tox [64, 65] and different molecular mechanisms have been proposed, including cell death as a cellular consequence to exaggerated RNase H activity resulting from non-selective hybridization [66, 67]. An alternative mechanism proposed involves PS backbone-dependent binding to key intracellular proteins in a sequence and chemistry-dependent manner. Being more hydrophobic, the higher affinity modifications showing higher incidence of liver toxicity also show higher affinity to a number of intracellular proteins compared to the same sequence with, e.g., MOE chemistry [68,69,70,71]. Importantly, predictive in vitro models for liver and kidney toxicity have been developed [67, 72, 73], and design modifications reducing ASO toxicity without compromising potency have been proposed [69, 74] demonstrating that highly potent ASO sequences that do not show liver toxicity can be identified and progressed to clinical trials.
Immune-stimulatory effects have long been a prominent feature of ONDs, where responses may vary widely between species and depend on oligonucleotide design and sequence, as well as chemical modifications [75,76,77,78,79,80,81,82,83,84,85]. The immunomodulatory potential can deliberately be used to design nucleotide-based immunotherapies and vaccine adjuvants, often harboring so-called CpG motifs [18,19,20,21], but for most other OND these effects are unwanted. Despite avoiding established CpG motifs in the design phase, some therapeutic oligos induce clear proinflammatory effects that can show in several different ways in the clinic, including injection site or infusion related reactions, flu-like symptoms and thrombocytopenia [86,87,88,89,90]. These effects are dose-dependent and can occur at different time points after first administration of the drug . Rodents are particularly sensitive to the immunostimulatory effects of ONDs [91, 92]. Similar to the liver toxicity described above, oligo sequence is a key parameter defining the proinflammatory property of therapeutic oligos and subtle and systematic sequence modifications to a proinflammatory ASO resulted in clear differences in proinflammatory potential [93]. Chemical modifications can modify the immune stimulatory potential of ONDs of a given sequence: PS modification of the backbone has long been known to increase the immune stimulatory properties of ONDs [80, 85, 94], whereas the neutral backbone in PMOs does not evoke an immune response [95]. 5′-methylation of cytosine is frequently used to suppress the immune stimulatory effect of CpG DNA sequences [76, 79]. 2′OMe modification of ssRNA or siRNA sequences inhibit immune stimulation, whereby even single modifications can significantly reduce the cytokine upregulation [78, 84]. Other 2′ribose modifications (2′F, 2′H, 2′MOE, LNA) have also been described to reduce proinflammatory effects [76, 83]. Therapeutic oligos administered in lipid formulations have been shown to induce inflammatory responses, and humans seem to be more sensitive to these effects than both rodents and NHPs [96,97,98].
Thrombocytopenia (TCP), i.e., low concentration of circulating platelets, has been observed in NHP toxicity studies with ASOs. In most cases, the platelet counts show around 30% reduction from baseline and then stabilize at a non-adverse level. However, in some drug programs, a few individual monkeys have experienced severe TCP [99]. Severe TCP was observed in the phase 3 studies for volanesorsen and inotersen as well as for drisapersen [86, 100, 101]. These TCP events occurred in the highest dose group, and platelet counts increased after drug cessation. A combination of high dose of proinflammatory ASOs and possibly patient susceptibility factors seem to be the most likely cause: a high frequency of severe TCP in cynomolgus monkeys of Mauritian origin whereas no cases of severe TCP were observed when the same ASO was given to non-Mauritian cynomolgus monkeys in a follow-up study [102].
3.2 Development Phase: In-Depth Characterization and Documentation of the Oligo Candidate
3.2.1 Preclinical Safety Assessment During the Development Phase
Strategies for preclinical safety assessment of therapeutic oligos have been discussed elsewhere [22, 89]. Results from a survey across 23 companies developing therapeutic oligos performed in 2018 showed that most companies follow the two species small molecule approach as outlined by the ICH M3(R2) guideline [103]. Although a guideline recently was adopted by Japanese regulators, most health authorities lack formal regulatory guidelines for therapeutic oligos, so the expectations and experience may vary between regions and even within health authorities [103]. However, white papers published by cross-pharma groups like OSWG (Oligo Safety Working Group) on best practice recommendations are frequently used as informal guidelines [45, 51, 96, 104,105,106,107,108,109,110].
For the common situation with a human active candidate having limited cross-species activity for meaningful assessment of potential on-target toxicity, a surrogate molecule can be included in parallel to the clinical candidate. Such surrogate molecules should be of the same design and chemistry as the clinical candidate and have a good general safety profile to allow meaningful assessment and documentation of potential on-target toxicities. Such surrogate molecules are mostly designed to be active in the rodent species of choice.
Despite lack of positive results in regulatory genotoxicity studies as discussed in the OSWG white paper by Berman et al. [105], several health authorities still request in vitro and in vivo assessment of genotoxicity [103].
For small molecules, in vitro and in vivo safety pharmacology studies are important to rule out adverse functional effects on key organs such as the CNS, cardiovascular and respiratory systems. Safety pharmacology assessment has been discussed in another OSWG white paper [106]. To the knowledge of the author, there is no information on systemically administered ASOs or siRNA showing activity in vitro or in vivo safety pharmacology studies, including activity on the hERG channel or any other ion channels important for cardiovascular or neuronal function. However, direct delivery into heart and CNS is a different story and could result in functional effects.
Similar to small molecules, the general toxicity studies for ASOs and siRNA are performed in a rodent and a non-rodent species. The duration of these Good Laboratory Practice (GLP) studies range from 1 to 3 months in the beginning of a program to chronic studies of 6 and 9 months in duration for rodents and non-rodents, respectively. For double-stranded siRNA and microRNA mimics, the rat is by far the most commonly used rodent species whereas the mouse is rodent species of choice for most single stranded PS backbone ASO candidates [103]. Although rat is the most common rodent for toxicity studies of small molecules, rat specific lesions like Chronic Progressive Nephropathy (CPN) [91, 92] are aggravated by the high kidney concentrations resulting from systemic delivery of PS backbone ASO. CPN is of no human relevance [111] but can become problematic in toxicity studies of long duration.
Due to the highest likelihood of sequence-dependent crossover on activity and a robust historical background record, the non-human primate (NHP), is by far the most common non-rodent species used for both ASO and siRNA candidates, but other non-rodents have been evaluated [103], including the pig [112]. Other in vivo studies required before regulatory approval include developmental and reproductive toxicology studies (DART) and carcinogenicity studies. Considerations for the DART studies are described in an OSWG white paper [108] and is normally run in mouse , rat or rabbit. Carcinogenicity studies are commonly run as a lifelong (2 years) studies in rat and mice or a 2-year study in rat combined with a 6-month study in a transgenic mouse model. The relevance of these carcinogenicity studies for ASOs and siRNA has been questioned, but most health authorities are likely waiting for more data before discussing whether these studies can get a waiver or not.
3.2.2 Regulatory Perspective
Several of the oligonucleotide products approved to date (e.g., eteplirsen, mipomersen, inotersen, volanesorsen) are aimed at treating rare, often genetic diseases for which no alternative treatment is available. In such cases, the presence of some safety signals has been judged to be acceptable. However, with some recent projects aiming at targeting significantly larger populations with more common disease, like the cholesterol-lowering siRNA inclisiran targeting PCSK9 and for which other treatments exist, the risk:benefit assessment will likely be different. At the same time as ONDs are considered for much larger and broader patient populations than before, exciting opportunities on the other end of the patient population spectrum are emerging. Milasen is a splice modulating ASO developed to treat a fatal neurodegenerative condition unique to a single patient [113]. With increasingly refined and optimized screening cascades, OND treatments for N = 1 and other ultrarare conditions will most likely become more common practice.
In summary, safety of ONDs depend on sequence, chemistry , design , and delivery approach. Several properties like limited species cross-reactivity, long tissue half-life and restricted productive uptake distribution needs to be considered when designing interpreting results for hybridization dependent on – and off-target safety assessment studies. Together with liver and kidney toxicity, proinflammatory effects are the most commonly observed safety findings in preclinical studies. With improved mechanistic understanding and screening approaches, more potent OND candidates with better safety profile can be identified for treatment of an increasing range of diseases and patient populations.
References
Crooke ST, Witztum JL, Bennett CF, Baker BF (2018) RNA-targeted therapeutics. Cell Metab 27(4):714–739. https://doi.org/10.1016/j.cmet.2018.03.004
Zhang MM, Bahal R, Rasmussen TP, Manautou JE, Zhong XB (2021) The growth of siRNA-based therapeutics: updated clinical studies. Biochem Pharmacol 189:114432. https://doi.org/10.1016/j.bcp.2021.114432
Khvorova A, Watts JK (2017) The chemical evolution of oligonucleotide therapies of clinical utility. Nat Biotechnol 35(3):238–248. https://doi.org/10.1038/nbt.3765
Seth PP, Swayze EE (2014) Unnatural nucleoside analoges for antisense therapy. In: Hanessian S (ed) Natural products in medicinal chemistry, 1st edn. Wiley-VCH Verlag, Weinheim, pp 403–439
Wan WB, Seth PP (2016) The medicinal chemistry of therapeutic oligonucleotides. J Med Chem 59(21):9645–9667. https://doi.org/10.1021/acs.jmedchem.6b00551
Bennett CF (2019) Therapeutic antisense oligonucleotides are coming of age. Annu Rev Med 70:307–321. https://doi.org/10.1146/annurev-med-041217-010829
Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M (2005) Silencing of microRNAs in vivo with ‘antagomirs’. Nature 438(7068):685–689. https://doi.org/10.1038/nature04303
Rupaimoole R, Slack FJ (2017) MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat Rev Drug Discov 16(3):203–222. https://doi.org/10.1038/nrd.2016.246
Carver MP, Charleston JS, Shanks C, Zhang J, Mense M, Sharma AK, Kaur H, Sazani P (2016) Toxicological characterization of exon skipping Phosphorodiamidate Morpholino oligomers (PMOs) in non-human primates. J Neuromusc Dis 3(3):381–393. https://doi.org/10.3233/JND-160157
Goyenvalle A, Leumann C, Garcia L (2016) Therapeutic potential of tricyclo-DNA antisense oligonucleotides. J Neuromusc Dis 3(2):157–167. https://doi.org/10.3233/JND-160146
Hammond SM, Hazell G, Shabanpoor F, Saleh AF, Bowerman M, Sleigh JN, Meijboom KE, Zhou H, Muntoni F, Talbot K, Gait MJ, Wood MJ (2016) Systemic peptide-mediated oligonucleotide therapy improves long-term survival in spinal muscular atrophy. Proc Natl Acad Sci U S A 113(39):10962–10967. https://doi.org/10.1073/pnas.1605731113
Montazersaheb S, Hejazi MS, Nozad Charoudeh H (2018) Potential of peptide nucleic acids in future therapeutic applications. Adv Pharmaceut Bull 8(4):551–563. https://doi.org/10.15171/apb.2018.064
Saleh AF, Arzumanov AA, Gait MJ (2012) Overview of alternative oligonucleotide chemistries for exon skipping. Methods Mol Biol 867:365–378. https://doi.org/10.1007/978-1-61779-767-5_23
Titze-de-Almeida R, David C, Titze-de-Almeida SS (2017) The race of 10 synthetic RNAi-based drugs to the pharmaceutical market. Pharm Res 34(7):1339–1363. https://doi.org/10.1007/s11095-017-2134-2
Bobbin ML, Rossi JJ (2016) RNA interference (RNAi)-based therapeutics: delivering on the promise? Annu Rev Pharmacol Toxicol 56:103–122. https://doi.org/10.1146/annurev-pharmtox-010715-103633
Nimjee SM, White RR, Becker RC, Sullenger BA (2017) Aptamers as therapeutics. Annu Rev Pharmacol Toxicol 57:61–79. https://doi.org/10.1146/annurev-pharmtox-010716-104558
Zhou J, Rossi J (2017) Aptamers as targeted therapeutics: current potential and challenges. Nat Rev Drug Discov 16(3):181–202. https://doi.org/10.1038/nrd.2016.199
Jackson S, Candia AF, Delaney S, Floettmann S, Wong C, Campbell JD, Kell S, Lum J, Hessel EM, Traquina P, McHale M, Robinson I, Bell J, Fuhr R, Keeling D, Coffman RL (2018) First-in-human study with the inhaled TLR9 oligonucleotide agonist AZD1419 results in interferon responses in the lung, and is safe and well-tolerated. Clin Pharmacol Ther 104(2):335–345. https://doi.org/10.1002/cpt.938
Krieg AM (2006) Therapeutic potential of toll-like receptor 9 activation. Nat Rev Drug Discov 5(6):471–484. https://doi.org/10.1038/nrd2059
Holtick U, Scheulen ME, von Bergwelt-Baildon MS, Weihrauch MR (2011) Toll-like receptor 9 agonists as cancer therapeutics. Expert Opin Investig Drugs 20(3):361–372. https://doi.org/10.1517/13543784.2011.553187
Vollmer J, Krieg AM (2009) Immunotherapeutic applications of CpG oligodeoxynucleotide TLR9 agonists. Adv Drug Deliv Rev 61(3):195–204. https://doi.org/10.1016/j.addr.2008.12.008
Andersson P, Den Besten C (2019) Preclinical and clinical drug-metabolism, pharmacokinetics and safety of therapeutic oligonucleotides. In: Agrawal S, Gait MJ (eds) Advances in nucleic acid therapeutics. Drug discovery series, 1st edn. Royal Society of Chemistry, London, pp 474–531
Crooke ST, Wang S, Vickers TA, Shen W, Liang XH (2017) Cellular uptake and trafficking of antisense oligonucleotides. Nat Biotechnol 35(3):230–237. https://doi.org/10.1038/nbt.3779
Koller E, Vincent TM, Chappell A, De S, Manoharan M, Bennett CF (2011) Mechanisms of single-stranded phosphorothioate modified antisense oligonucleotide accumulation in hepatocytes. Nucleic Acids Res 39(11):4795–4807. https://doi.org/10.1093/nar/gkr089
Hung G, Xiao X, Peralta R, Bhattacharjee G, Murray S, Norris D, Guo S, Monia BP (2013) Characterization of target mRNA reduction through in situ RNA hybridization in multiple organ systems following systemic antisense treatment in animals. Nucl Acid Ther 23(6):369–378. https://doi.org/10.1089/nat.2013.0443
Kulkarni JA, Cullis PR, van der Meel R (2018) Lipid nanoparticles enabling gene therapies: from concepts to clinical utility. Nucl Acid Ther 28(3):146–157. https://doi.org/10.1089/nat.2018.0721
Khorkova O, Wahlestedt C (2017) Oligonucleotide therapies for disorders of the nervous system. Nat Biotechnol 35(3):249–263. https://doi.org/10.1038/nbt.3784
Querbes W, Ge P, Zhang W, Fan Y, Costigan J, Charisse K, Maier M, Nechev L, Manoharan M, Kotelianski V, Sah DW (2009) Direct CNS delivery of siRNA mediates robust silencing in oligodendrocytes. Oligonucleotides 19(1):23–29. https://doi.org/10.1089/oli.2008.0165
Rigo F, Chun SJ, Norris DA, Hung G, Lee S, Matson J, Fey RA, Gaus H, Hua Y, Grundy JS, Krainer AR, Henry SP, Bennett CF (2014) Pharmacology of a central nervous system delivered 2′-O-methoxyethyl-modified survival of motor neuron splicing oligonucleotide in mice and nonhuman primates. J Pharmacol Exp Ther 350(1):46–55. https://doi.org/10.1124/jpet.113.212407
Henry SP, Miner RC, Drew WL, Fitchett J, York-Defalco C, Rapp LM, Levin AA (2001) Antiviral activity and ocular kinetics of antisense oligonucleotides designed to inhibit CMV replication. Invest Ophthalmol Vis Sci 42(11):2646–2651
Danis RP, Henry SP, Ciulla TA (2001) Potential therapeutic application of antisense oligonucleotides in the treatment of ocular diseases. Expert Opin Pharmacother 2(2):277–291. https://doi.org/10.1517/14656566.2.2.277
Leeds JM, Henry SP, Bistner S, Scherrill S, Williams K, Levin AA (1998) Pharmacokinetics of an antisense oligonucleotide injected intravitreally in monkeys. Drug Metab Dispos 26(7):670–675
Solano EC, Kornbrust DJ, Beaudry A, Foy JW, Schneider DJ, Thompson JD (2014) Toxicological and pharmacokinetic properties of QPI-1007, a chemically modified synthetic siRNA targeting caspase 2 mRNA, following intravitreal injection. Nucl Acid Ther 24(4):258–266. https://doi.org/10.1089/nat.2014.0489
Dowdy SF (2017) Overcoming cellular barriers for RNA therapeutics. Nat Biotechnol 35(3):222–229. https://doi.org/10.1038/nbt.3802
Juliano R, Alam MR, Dixit V, Kang H (2008) Mechanisms and strategies for effective delivery of antisense and siRNA oligonucleotides. Nucleic Acids Res 36(12):4158–4171. https://doi.org/10.1093/nar/gkn342
Juliano RL (2016) The delivery of therapeutic oligonucleotides. Nucleic Acids Res 44(14):6518–6548. https://doi.org/10.1093/nar/gkw236
Juliano RL, Alahari S, Yoo H, Kole R, Cho M (1999) Antisense pharmacodynamics: critical issues in the transport and delivery of antisense oligonucleotides. Pharm Res 16(4):494–502
Godfrey C, Desviat LR, Smedsrod B, Pietri-Rouxel F, Denti MA, Disterer P, Lorain S, Nogales-Gadea G, Sardone V, Anwar R, El Andaloussi S, Lehto T, Khoo B, Brolin C, van Roon-Mom WM, Goyenvalle A, Aartsma-Rus A, Arechavala-Gomeza V (2017) Delivery is key: lessons learnt from developing splice-switching antisense therapies. EMBO Mol Med 9(5):545–557. https://doi.org/10.15252/emmm.201607199
Hammond SM, Aartsma-Rus A, Alves S, Borgos SE, Buijsen RAM, Collin RWJ, Covello G, Denti MA, Desviat LR, Echevarría L, Foged C, Gaina G, Garanto A, Goyenvalle AT, Guzowska M, Holodnuka I, Jones DR, Krause S, Lehto T, Montolio M, Van Roon-Mom W, Arechavala-Gomeza V (2021) Delivery of oligonucleotide-based therapeutics: challenges and opportunities. EMBO Mol Med 13(4):e13243. https://doi.org/10.15252/emmm.202013243
Ledford H (2018) Gene-silencing technology gets first drug approval after 20-year wait. Nature 560(7718):291–292. https://doi.org/10.1038/d41586-018-05867-7
Coelho T, Adams D, Silva A, Lozeron P, Hawkins PN, Mant T, Perez J, Chiesa J, Warrington S, Tranter E, Munisamy M, Falzone R, Harrop J, Cehelsky J, Bettencourt BR, Geissler M, Butler JS, Sehgal A, Meyers RE, Chen Q, Borland T, Hutabarat RM, Clausen VA, Alvarez R, Fitzgerald K, Gamba-Vitalo C, Nochur SV, Vaishnaw AK, Sah DW, Gollob JA, Suhr OB (2013) Safety and efficacy of RNAi therapy for transthyretin amyloidosis. N Engl J Med 369(9):819–829. https://doi.org/10.1056/NEJMoa1208760
Nair JK, Willoughby JL, Chan A, Charisse K, Alam MR, Wang Q, Hoekstra M, Kandasamy P, Kel’in AV, Milstein S, Taneja N, O’Shea J, Shaikh S, Zhang L, van der Sluis RJ, Jung ME, Akinc A, Hutabarat R, Kuchimanchi S, Fitzgerald K, Zimmermann T, van Berkel TJ, Maier MA, Rajeev KG, Manoharan M (2014) Multivalent N-acetylgalactosamine-conjugated siRNA localizes in hepatocytes and elicits robust RNAi-mediated gene silencing. J Am Chem Soc 136(49):16958–16961. https://doi.org/10.1021/ja505986a
Prakash TP, Graham MJ, Yu J, Carty R, Low A, Chappell A, Schmidt K, Zhao C, Aghajan M, Murray HF, Riney S, Booten SL, Murray SF, Gaus H, Crosby J, Lima WF, Guo S, Monia BP, Swayze EE, Seth PP (2014) Targeted delivery of antisense oligonucleotides to hepatocytes using triantennary N-acetyl galactosamine improves potency 10-fold in mice. Nucleic Acids Res 42(13):8796–8807. https://doi.org/10.1093/nar/gku531
Crooke ST, Baker BF, Xia S, Yu RZ, Viney NJ, Wang Y, Tsimikas S, Geary RS (2019) Integrated assessment of the clinical performance of GalNAc3-conjugated 2′-O-Methoxyethyl chimeric antisense oligonucleotides: I. Human volunteer experience. Nucl Acid Ther 29(1):16–32. https://doi.org/10.1089/nat.2018.0753
Kornbrust D, Cavagnaro J, Levin A, Foy J, Pavco P, Gamba-Vitalo C, Guimond A (2013) Oligo safety working group exaggerated pharmacology subcommittee consensus document. Nucl Acid Ther 23(1):21–28. https://doi.org/10.1089/nat.2012.0399
Hagedorn PH, Hansen BR, Koch T, Lindow M (2017) Managing the sequence-specificity of antisense oligonucleotides in drug discovery. Nucleic Acids Res 45(5):2262–2282. https://doi.org/10.1093/nar/gkx056
Jackson AL, Linsley PS (2010) Recognizing and avoiding siRNA off-target effects for target identification and therapeutic application. Nat Rev Drug Discov 9(1):57–67. https://doi.org/10.1038/nrd3010
Janas MM, Schlegel MK, Harbison CE, Yilmaz VO, Jiang Y, Parmar R, Zlatev I, Castoreno A, Xu H, Shulga-Morskaya S, Rajeev KG, Manoharan M, Keirstead ND, Maier MA, Jadhav V (2018) Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat Commun 9(1):723. https://doi.org/10.1038/s41467-018-02989-4
Kamola PJ, Kitson JD, Turner G, Maratou K, Eriksson S, Panjwani A, Warnock LC, Douillard Guilloux GA, Moores K, Koppe EL, Wixted WE, Wilson PA, Gooderham NJ, Gant TW, Clark KL, Hughes SA, Edbrooke MR, Parry JD (2015) In silico and in vitro evaluation of exonic and intronic off-target effects form a critical element of therapeutic ASO gapmer optimization. Nucleic Acids Res 43(18):8638–8650. https://doi.org/10.1093/nar/gkv857
Watt AT, Swayze G, Swayze EE, Freier SM (2020) Likelihood of nonspecific activity of Gapmer antisense oligonucleotides is associated with relative hybridization free energy. Nucl Acid Ther 30(4):215–228. https://doi.org/10.1089/nat.2020.0847
Lindow M, Vornlocher HP, Riley D, Kornbrust DJ, Burchard J, Whiteley LO, Kamens J, Thompson JD, Nochur S, Younis H, Bartz S, Parry J, Ferrari N, Henry SP, Levin AA (2012) Assessing unintended hybridization-induced biological effects of oligonucleotides. Nat Biotechnol 30(10):920–923. https://doi.org/10.1038/nbt.2376
Ray KK, Landmesser U, Leiter LA, Kallend D, Dufour R, Karakas M, Hall T, Troquay RP, Turner T, Visseren FL, Wijngaard P, Wright RS, Kastelein JJ (2017) Inclisiran in patients at high cardiovascular risk with elevated LDL cholesterol. N Engl J Med 376(15):1430–1440. https://doi.org/10.1056/NEJMoa1615758
Engelhardt JA, Fant P, Guionaud S, Henry SP, Leach MW, Louden C, Scicchitano MS, Weaver JL, Zabka TS, Frazier KS, Society of Toxicologic Pathology Vascular Injury Working G (2015) Scientific and regulatory policy committee points-to-consider paper*: drug-induced vascular injury associated with nonsmall molecule therapeutics in preclinical development: part 2. Antisense oligonucleotides. Toxicol Pathol 43(7):935–944. https://doi.org/10.1177/0192623315570341
Henry SP, Jagels MA, Hugli TE, Manalili S, Geary RS, Giclas PC, Levin AA (2014) Mechanism of alternative complement pathway dysregulation by a phosphorothioate oligonucleotide in monkey and human serum. Nucl Acid Ther 24(5):326–335. https://doi.org/10.1089/nat.2014.0491
Shen L, Frazer-Abel A, Reynolds PR, Giclas PC, Chappell A, Pangburn MK, Younis H, Henry SP (2014) Mechanistic understanding for the greater sensitivity of monkeys to antisense oligonucleotide-mediated complement activation compared with humans. J Pharmacol Exp Ther 351(3):709–717. https://doi.org/10.1124/jpet.114.219378
Bosgra S, Sipkens J, de Kimpe S, den Besten C, Datson N, van Deutekom J (2019) The pharmacokinetics of 2′-O-methyl Phosphorothioate antisense oligonucleotides: experiences from developing exon skipping therapies for Duchenne muscular dystrophy. Nucl Acid Ther 29(6):305–322. https://doi.org/10.1089/nat.2019.0805
Geary RS, Norris D, Yu R, Bennett CF (2015) Pharmacokinetics, biodistribution and cell uptake of antisense oligonucleotides. Adv Drug Deliv Rev 87:46–51. https://doi.org/10.1016/j.addr.2015.01.008
Levin AA, Yu RZ, Geary RS (2008) Basic principles of the pharmacokinetics of antisense oligonucleotide drugs. In: Crooke ST (ed) Antisense drug technology—principles, strategies, and applications, 2nd edn. CRC Press, Taylor and Francis Group, Boca Raton, FL, pp 183–215
Yu RZ, Kim TW, Hong A, Watanabe TA, Gaus HJ, Geary RS (2007) Cross-species pharmacokinetic comparison from mouse to man of a second-generation antisense oligonucleotide, ISIS 301012, targeting human apolipoprotein B-100. Drug Metab Dispos 35(3):460–468. https://doi.org/10.1124/dmd.106.012401
Yu RZ, Lemonidis KM, Graham MJ, Matson JE, Crooke RM, Tribble DL, Wedel MK, Levin AA, Geary RS (2009) Cross-species comparison of in vivo PK/PD relationships for second-generation antisense oligonucleotides targeting apolipoprotein B-100. Biochem Pharmacol 77(5):910–919. https://doi.org/10.1016/j.bcp.2008.11.005
Crooke ST, Baker BF, Kwoh TJ, Cheng W, Schulz DJ, Xia S, Salgado N, Bui HH, Hart CE, Burel SA, Younis HS, Geary RS, Henry SP, Bhanot S (2016) Integrated safety assessment of 2′-O-Methoxyethyl chimeric antisense oligonucleotides in NonHuman primates and healthy human volunteers. Mol Ther 24(10):1771–1782. https://doi.org/10.1038/mt.2016.136
Shen L, Engelhardt JA, Hung G, Yee J, Kikkawa R, Matson J, Tayefeh B, Machemer T, Giclas PC, Henry SP (2016) Effects of repeated complement activation associated with chronic treatment of Cynomolgus monkeys with 2′-O-Methoxyethyl modified antisense oligonucleotide. Nucl Acid Ther 26(4):236–249. https://doi.org/10.1089/nat.2015.0584
Hagedorn PH, Persson R, Funder ED, Albaek N, Diemer SL, Hansen DJ, Moller MR, Papargyri N, Christiansen H, Hansen BR, Hansen HF, Jensen MA, Koch T (2018) Locked nucleic acid: modality, diversity, and drug discovery. Drug Discov Today 23(1):101–114. https://doi.org/10.1016/j.drudis.2017.09.018
Burdick AD, Sciabola S, Mantena SR, Hollingshead BD, Stanton R, Warneke JA, Zeng M, Martsen E, Medvedev A, Makarov SS, Reed LA, Davis JW 2nd, Whiteley LO (2014) Sequence motifs associated with hepatotoxicity of locked nucleic acid—modified antisense oligonucleotides. Nucleic Acids Res 42(8):4882–4891. https://doi.org/10.1093/nar/gku142
Hagedorn PH, Yakimov V, Ottosen S, Kammler S, Nielsen NF, Hog AM, Hedtjarn M, Meldgaard M, Moller MR, Orum H, Koch T, Lindow M (2013) Hepatotoxic potential of therapeutic oligonucleotides can be predicted from their sequence and modification pattern. Nucl Acid Ther 23(5):302–310. https://doi.org/10.1089/nat.2013.0436
Burel SA, Hart CE, Cauntay P, Hsiao J, Machemer T, Katz M, Watt A, Bui HH, Younis H, Sabripour M, Freier SM, Hung G, Dan A, Prakash TP, Seth PP, Swayze EE, Bennett CF, Crooke ST, Henry SP (2016) Hepatotoxicity of high affinity gapmer antisense oligonucleotides is mediated by RNase H1 dependent promiscuous reduction of very long pre-mRNA transcripts. Nucleic Acids Res 44(5):2093–2109. https://doi.org/10.1093/nar/gkv1210
Dieckmann A, Hagedorn PH, Burki Y, Brugmann C, Berrera M, Ebeling M, Singer T, Schuler F (2018) A sensitive in vitro approach to assess the hybridization-dependent toxic potential of high affinity Gapmer oligonucleotides. Mol Ther Nucl Acids 10:45–54. https://doi.org/10.1016/j.omtn.2017.11.004
Liang XH, Sun H, Shen W, Crooke ST (2015) Identification and characterization of intracellular proteins that bind oligonucleotides with phosphorothioate linkages. Nucleic Acids Res 43(5):2927–2945. https://doi.org/10.1093/nar/gkv143
Shen W, De Hoyos CL, Migawa MT, Vickers TA, Sun H, Low A, Bell TA 3rd, Rahdar M, Mukhopadhyay S, Hart CE, Bell M, Riney S, Murray SF, Greenlee S, Crooke RM, Liang XH, Seth PP, Crooke ST (2019) Chemical modification of PS-ASO therapeutics reduces cellular protein-binding and improves the therapeutic index. Nat Biotechnol 37(6):640–650. https://doi.org/10.1038/s41587-019-0106-2
Shen W, De Hoyos CL, Sun H, Vickers TA, Liang XH, Crooke ST (2018) Acute hepatotoxicity of 2′ fluoro-modified 5-10-5 gapmer phosphorothioate oligonucleotides in mice correlates with intracellular protein binding and the loss of DBHS proteins. Nucleic Acids Res 46(5):2204–2217. https://doi.org/10.1093/nar/gky060
Shen W, Liang XH, Sun H, Crooke ST (2015) 2′-Fluoro-modified phosphorothioate oligonucleotide can cause rapid degradation of P54nrb and PSF. Nucleic Acids Res 43(9):4569–4578. https://doi.org/10.1093/nar/gkv298
Moisan A, Gubler M, Zhang JD, Tessier Y, Dumong Erichsen K, Sewing S, Gerard R, Avignon B, Huber S, Benmansour F, Chen X, Villasenor R, Braendli-Baiocco A, Festag M, Maunz A, Singer T, Schuler F, Roth AB (2017) Inhibition of EGF uptake by nephrotoxic antisense drugs in vitro and implications for preclinical safety profiling. Mol Ther Nucl Acids 6:89–105. https://doi.org/10.1016/j.omtn.2016.11.006
Sewing S, Boess F, Moisan A, Bertinetti-Lapatki C, Minz T, Hedtjaern M, Tessier Y, Schuler F, Singer T, Roth AB (2016) Establishment of a predictive in vitro assay for assessment of the hepatotoxic potential of oligonucleotide drugs. PLoS One 11(7):e0159431. https://doi.org/10.1371/journal.pone.0159431
Vasquez G, Freestone GC, Wan WB, Low A, De Hoyos CL, Yu J, Prakash TP, Ostergaard ME, Liang XH, Crooke ST, Swayze EE, Migawa MT, Seth PP (2021) Site-specific incorporation of 5′-methyl DNA enhances the therapeutic profile of gapmer ASOs. Nucleic Acids Res 49(4):1828–1839. https://doi.org/10.1093/nar/gkab047
Hamm S, Latz E, Hangel D, Muller T, Yu P, Golenbock D, Sparwasser T, Wagner H, Bauer S (2010) Alternating 2′-O-ribose methylation is a universal approach for generating non-stimulatory siRNA by acting as TLR7 antagonist. Immunobiology 215(7):559–569. https://doi.org/10.1016/j.imbio.2009.09.003
Henry S, Stecker K, Brooks D, Monteith D, Conklin B, Bennett CF (2000) Chemically modified oligonucleotides exhibit decreased immune stimulation in mice. J Pharmacol Exp Ther 292(2):468–479
Hornung V, Ellegast J, Kim S, Brzozka K, Jung A, Kato H, Poeck H, Akira S, Conzelmann KK, Schlee M, Endres S, Hartmann G (2006) 5′-triphosphate RNA is the ligand for RIG-I. Science 314(5801):994–997. https://doi.org/10.1126/science.1132505
Judge AD, Sood V, Shaw JR, Fang D, McClintock K, MacLachlan I (2005) Sequence-dependent stimulation of the mammalian innate immune response by synthetic siRNA. Nat Biotechnol 23(4):457–462. https://doi.org/10.1038/nbt1081
Krieg AM (2002) CpG motifs in bacterial DNA and their immune effects. Annu Rev Immunol 20:709–760. https://doi.org/10.1146/annurev.immunol.20.100301.064842
Liang H, Nishioka Y, Reich CF, Pisetsky DS, Lipsky PE (1996) Activation of human B cells by phosphorothioate oligodeoxynucleotides. J Clin Invest 98(5):1119–1129. https://doi.org/10.1172/JCI118894
Robbins M, Judge A, Liang L, McClintock K, Yaworski E, MacLachlan I (2007) 2′-O-methyl-modified RNAs act as TLR7 antagonists. Mol Ther 15(9):1663–1669. https://doi.org/10.1038/sj.mt.6300240
Sewing S, Roth AB, Winter M, Dieckmann A, Bertinetti-Lapatki C, Tessier Y, McGinnis C, Huber S, Koller E, Ploix C, Reed JC, Singer T, Rothfuss A (2017) Assessing single-stranded oligonucleotide drug-induced effects in vitro reveals key risk factors for thrombocytopenia. PLoS One 12(11):e0187574. https://doi.org/10.1371/journal.pone.0187574
Sioud M (2006) Single-stranded small interfering RNA are more immunostimulatory than their double-stranded counterparts: a central role for 2′-hydroxyl uridines in immune responses. Eur J Immunol 36(5):1222–1230. https://doi.org/10.1002/eji.200535708
Tluk S, Jurk M, Forsbach A, Weeratna R, Samulowitz U, Krieg AM, Bauer S, Vollmer J (2009) Sequences derived from self-RNA containing certain natural modifications act as suppressors of RNA-mediated inflammatory immune responses. Int Immunol 21(5):607–619. https://doi.org/10.1093/intimm/dxp030
Zhao Q, Temsamani J, Iadarola PL, Jiang Z, Agrawal S (1996) Effect of different chemically modified oligodeoxynucleotides on immune stimulation. Biochem Pharmacol 51(2):173–182
Chi X, Gatti P, Papoian T (2017) Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov Today 22(5):823–833. https://doi.org/10.1016/j.drudis.2017.01.013
Goemans NM, Tulinius M, van den Hauwe M, Kroksmark AK, Buyse G, Wilson RJ, van Deutekom JC, de Kimpe SJ, Lourbakos A, Campion G (2016) Long-term efficacy, safety, and pharmacokinetics of Drisapersen in Duchenne muscular dystrophy: results from an open-label extension study. PLoS One 11(9):e0161955. https://doi.org/10.1371/journal.pone.0161955
Kwoh TJ (2008) An overview of the clinical safety experience of first-and second-generation antisense oligonucleotides. In: Crooke ST (ed) Antisense drug technology: principles, strategies and applications, 2nd edn. CRC Press, Carlsbad, CA, pp 365–399
Mustonen EK, Palomaki T, Pasanen M (2017) Oligonucleotide-based pharmaceuticals: non-clinical and clinical safety signals and non-clinical testing strategies. Regul Toxicol Pharmacol 90:328–341. https://doi.org/10.1016/j.yrtph.2017.09.028
van Meer L, Moerland M, Gallagher J, van Doorn MB, Prens EP, Cohen AF, Rissmann R, Burggraaf J (2016) Injection site reactions after subcutaneous oligonucleotide therapy. Br J Clin Pharmacol 82(2):340–351. https://doi.org/10.1111/bcp.12961
Frazier KS (2015) Antisense oligonucleotide therapies: the promise and the challenges from a toxicologic pathologist’s perspective. Toxicol Pathol 43(1):78–89. https://doi.org/10.1177/0192623314551840
Henry SP, Kim T-W, Kramer-Stickland K, Zanardi TA, Fey RA, Levin AA (2008) Toxicologic properties of 2′-methoxyethyl chimeric antisense inhibitors in animals and man. In: Crooke ST (ed) Antisense drug technology: principles, strategies and applications, 2nd edn. CRC Press, Taylor and Francis Group, Boca Raton, FL, pp 327–363
Paz S, Hsiao J, Cauntay P, Soriano A, Bai L, Machemer T, Xiao X, Guo S, Hung G, Younis H, Bennett CF, Henry S, Yun TJ, Burel S (2017) The distinct and cooperative roles of toll-like receptor 9 and receptor for advanced glycation end products in modulating in vivo inflammatory responses to select CpG and non-CpG oligonucleotides. Nucl Acid Ther 27(5):272–284. https://doi.org/10.1089/nat.2017.0668
Jeske S, Pries R, Wollenberg B (2013) CpG-induced IFN-alpha production of plasmacytoid dendritic cells: time and dosage dependence and the effect of structural modifications to the CpG backbone. Nucl Acid Ther 23(2):118–124. https://doi.org/10.1089/nat.2012.0384
Iversen PL (2008) Morpholinos. In: Crooke ST (ed) Antisense drug technology: principles, strategies and applications, 2nd edn. CRC Press, Carlsbad, CA, pp 565–582
Marlowe JL, Akopian V, Karmali P, Kornbrust D, Lockridge J, Semple S (2017) Recommendations of the oligonucleotide safety working Group’s formulated oligonucleotide subcommittee for the Safety Assessment of formulated oligonucleotide-based therapeutics. Nucl Acid Ther 27(4):183–196. https://doi.org/10.1089/nat.2017.0671
Suhr OB, Coelho T, Buades J, Pouget J, Conceicao I, Berk J, Schmidt H, Waddington-Cruz M, Campistol JM, Bettencourt BR, Vaishnaw A, Gollob J, Adams D (2015) Efficacy and safety of patisiran for familial amyloidotic polyneuropathy: a phase II multi-dose study. Orphanet J Rare Dis 10:109. https://doi.org/10.1186/s13023-015-0326-6
Tabernero J, Shapiro GI, LoRusso PM, Cervantes A, Schwartz GK, Weiss GJ, Paz-Ares L, Cho DC, Infante JR, Alsina M, Gounder MM, Falzone R, Harrop J, White AC, Toudjarska I, Bumcrot D, Meyers RE, Hinkle G, Svrzikapa N, Hutabarat RM, Clausen VA, Cehelsky J, Nochur SV, Gamba-Vitalo C, Vaishnaw AK, Sah DW, Gollob JA, Burris HA 3rd (2013) First-in-humans trial of an RNA interference therapeutic targeting VEGF and KSP in cancer patients with liver involvement. Cancer Discov 3(4):406–417. https://doi.org/10.1158/2159-8290.CD-12-0429
Henry SP, Narayanan P, Shen L, Bhanot S, Younis HS, Burel SA (2017) Assessment of the effects of 2′-Methoxyethyl antisense oligonucleotides on platelet count in Cynomolgus nonhuman primates. Nucl Acid Ther 27(4):197–208. https://doi.org/10.1089/nat.2017.0666
Crooke ST, Baker BF, Witztum JL, Kwoh TJ, Pham NC, Salgado N, McEvoy BW, Cheng W, Hughes SG, Bhanot S, Geary RS (2017) The effects of 2′-O-Methoxyethyl containing antisense oligonucleotides on platelets in human clinical trials. Nucl Acid Ther 27(3):121–129. https://doi.org/10.1089/nat.2016.0650
EMA (2018) Kynamro assessment report. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Public_assessment_report/human/002429/WC500144511pdf
Andersson P, den Besten C (2019) Chapter 20. Preclinical and clinical drug-metabolism, pharmacokinetics and safety of therapeutic oligonucleotides. In: Advances in nucleic acid therapeutics. The Royal Society of Chemistry, London, pp 474–531. https://doi.org/10.1039/9781788015714-00474
Tessier Y, Achanzar W, Mihalcik L, Amuzie C, Andersson P, Parry JD, Moggs J, Whiteley LO (2021) Outcomes of the European Federation of Pharmaceutical Industries and Associations Oligonucleotide Working Group Survey on nonclinical practices and regulatory expectations for therapeutic oligonucleotide safety assessment. Nucl Acid Ther 31(1):7–20. https://doi.org/10.1089/nat.2020.0892
Alton EW, Boushey HA, Garn H, Green FH, Hodges M, Martin RJ, Murdoch RD, Renz H, Shrewsbury SB, Seguin R, Johnson G, Parry JD, Tepper J, Renzi P, Cavagnaro J, Ferrari N (2012) Clinical expert panel on monitoring potential lung toxicity of inhaled oligonucleotides: consensus points and recommendations. Nucl Acid Ther 22(4):246–254. https://doi.org/10.1089/nat.2012.0345
Berman CL, Barros SA, Galloway SM, Kasper P, Oleson FB, Priestley CC, Sweder KS, Schlosser MJ, Sobol Z (2016) OSWG recommendations for genotoxicity testing of novel oligonucleotide-based therapeutics. Nucl Acid Ther 26(2):73–85. https://doi.org/10.1089/nat.2015.0534
Berman CL, Cannon K, Cui Y, Kornbrust DJ, Lagrutta A, Sun SZ, Tepper J, Waldron G, Younis HS (2014) Recommendations for safety pharmacology evaluations of oligonucleotide-based therapeutics. Nucl Acid Ther 24(4):291–301. https://doi.org/10.1089/nat.2013.0477
Capaldi D, Teasdale A, Henry S, Akhtar N, den Besten C, Gao-Sheridan S, Kretschmer M, Sharpe N, Andrews B, Burm B, Foy J (2017) Impurities in oligonucleotide drug substances and drug products. Nucl Acid Ther 27(6):309–322. https://doi.org/10.1089/nat.2017.0691
Cavagnaro J, Berman C, Kornbrust D, White T, Campion S, Henry S (2014) Considerations for assessment of reproductive and developmental toxicity of oligonucleotide-based therapeutics. Nucl Acid Ther 24(5):313–325. https://doi.org/10.1089/nat.2014.0490
Henry SP, Seguin R, Cavagnaro J, Berman C, Tepper J, Kornbrust D (2016) Considerations for the characterization and interpretation of results related to alternative complement activation in monkeys associated with oligonucleotide-based therapeutics. Nucl Acid Ther 26(4):210–215. https://doi.org/10.1089/nat.2015.0593
Schubert D, Levin AA, Kornbrust D, Berman CL, Cavagnaro J, Henry S, Seguin R, Ferrari N, Shrewsbury SB (2012) The oligonucleotide safety working group (OSWG). Nucl Acid Ther 22(4):211–212. https://doi.org/10.1089/nat.2012.0383
Hard GC, Johnson KJ, Cohen SM (2009) A comparison of rat chronic progressive nephropathy with human renal disease-implications for human risk assessment. Crit Rev Toxicol 39(4):332–346. https://doi.org/10.1080/10408440802368642
Braendli-Baiocco A, Festag M, Dumong Erichsen K, Persson R, Mihatsch MJ, Fisker N, Funk J, Mohr S, Constien R, Ploix C, Brady K, Berrera M, Altmann B, Lenz B, Albassam M, Schmitt G, Weiser T, Schuler F, Singer T, Tessier Y (2017) From the cover: the Minipig is a suitable non-rodent model in the safety assessment of single stranded oligonucleotides. Toxicol Sci 157(1):112–128. https://doi.org/10.1093/toxsci/kfx025
Kim J, Hu C, Moufawad El Achkar C, Black LE, Douville J, Larson A, Pendergast MK, Goldkind SF, Lee EA, Kuniholm A, Soucy A, Vaze J, Belur NR, Fredriksen K, Stojkovska I, Tsytsykova A, Armant M, Di Donato RL, Choi J, Cornelissen L, Pereira LM, Augustine EF, Genetti CA, Dies K, Barton B, Williams L, Goodlett BD, Riley BL, Pasternak A, Berry ER, Pflock KA, Chu S, Reed C, Tyndall K, Agrawal PB, Beggs AH, Grant PE, Urion DK, Snyder RO, Waisbren SE, Poduri A, Park PJ, Patterson A, Biffi A, Mazzulli JR, Bodamer O, Berde CB, Yu TW (2019) Patient-customized oligonucleotide therapy for a rare genetic disease. N Engl J Med 381(17):1644–1652. https://doi.org/10.1056/NEJMoa1813279
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2022 The Author(s)
About this protocol
Cite this protocol
Andersson, P. (2022). Preclinical Safety Assessment of Therapeutic Oligonucleotides. In: Arechavala-Gomeza, V., Garanto, A. (eds) Antisense RNA Design, Delivery, and Analysis. Methods in Molecular Biology, vol 2434. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-2010-6_25
Download citation
DOI: https://doi.org/10.1007/978-1-0716-2010-6_25
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-2009-0
Online ISBN: 978-1-0716-2010-6
eBook Packages: Springer Protocols