Abstract
Renal diffusion-weighted imaging (DWI) can be used to obtain information on the microstructure of kidney tissue, and has the potential to provide MR-biomarkers for functional renal imaging. Here we describe in a step-by-step experimental protocol the MRI method for measuring renal diffusion coefficients in rodents using ADC or IVIM models. Both methods provide quantification of renal diffusion coefficients; however, IVIM, a more complex model, allows for the calculation of the pseudodiffusion and fraction introduced by tissue vascular and tubular components. DWI provides information of renal microstructure contributing to the understanding of the physiology and the underlying processes that precede the beginning of pathologies.
This chapter is based upon work from the COST Action PARENCHIMA, a community-driven network funded by the European Cooperation in Science and Technology (COST) program of the European Union, which aims to improve the reproducibility and standardization of renal MRI biomarkers. This experimental protocol chapter is complemented by two separate chapters describing the basic concept and data analysis.
You have full access to this open access chapter, Download protocol PDF
Similar content being viewed by others
Key words
1 Introduction
Diffusion weighting imaging (DWI ) is an MR imaging technique sensitive to the motion of water molecules within a voxel of tissue. The random movement, also known as Brownian motion, of fluid molecules makes them spread out until a boundary stops them. In general, extracellular water molecules will have a larger net displacement over time than intracellular molecules. Intracellular molecules have more chances to collide with cell walls, organelles, and macromolecules. Diseases and pathologies that influence tissue properties and structure may either increase or decrease the diffusion behavior of water in tissues, which can be quantified using DWI [1, 2].
In DWI , the image contrast is based on the displacement of water molecules. Voxels where molecules present a larger displacement during the diffusion-sensitization preparation will appear darker on the resulting image.
Sensitization to diffusion can be achieved by using an extra pair of magnetic field gradients during the pulse sequence , generating images with diffusion-weighting determined by the magnitude, duration, and separation of the gradients, which can be quantified by the acquisition parameter b-value [3].
The DWI signal decay across a series of images with different diffusion-weightings can then be analyzed, using more or less complex mathematical models, to quantify the diffusion parameters. Ideally, the acquisition should be designed considering the signal model one wishes to use. The simplest model considers only the apparent diffusion coefficient (ADC), an empirical parameter reflecting the overall water molecule motion in the tissue averaged over one or more directions using a small number of b-values. ADC can be estimated by fitting a monoexponential curve to the DWI signal as a function of b-value, and fitting signal curves on a pixel-by-pixel basis allows construction of diffusion parameter maps [4].
Perfusion in tissue introduces a pseudodiffusion component to the DWI signal, which cannot be quantified using the ADC model (but it will influence the calculated ADC if low b-values are included). A more complex model is needed to take separate out this effect. Intravoxel incoherent motion (IVIM) intends to model flow processes, which manifest as pseudodiffusion with a larger coefficient, by assuming the DWI signal as a function of the b-value is not monoexponential but biexponential. This method requires the acquisition of several b-values before the pseudodiffusion decays away, especially between b-values of 0 and 200 s/mm2 [5].
This experimental protocol chapter is complemented by two separate chapters describing the basic concept and data analysis, which are part of this book.
This chapter is part of the book Pohlmann A, Niendorf T (eds) (2020) Preclinical MRI of the Kidney—Methods and Protocols. Springer, New York.
2 Materials
2.1 Animals
This experimental protocol is tailored for mice (variants of C57BL/6) with a body mass of 15–35 g. Advice for adaptation to rats is given as Notes where necessary.
2.2 Lab Equipment and Chemicals
-
1.
Anesthesia: typically, 0.5–1.5% isoflurane is used for anesthesia administered to the mice using an anesthetic gas vaporizer (Leica Biosystems, Maryland, USA). For nonrecovery experiments, urethane solution (Sigma-Aldrich, Steinheim, Germany; 20% in distilled water) can provide anesthesia for several hours with comparatively fewer side effects on renal physiology, which is an important issue. For nonrecovery experiments urethane solution (Sigma-Aldrich, Steinheim, Germany; 20% in distilled water) can provide anesthesia for several hours with comparatively fewer side effects on renal physiology, which is an important issue. For an in-depth description and discussion of the anesthesia please refer to the chapter by Kaucsar T et al. “Preparation and Monitoring of Small Animals in Renal MRI.”
-
2.
Gases: O2, N2, and compressed air, as well as a gas-mixing system (FMI Föhr Medical Instruments GmbH, Seeheim-Ober Beerbach, Germany) or general inhalation anesthesia equipment, including an anesthetic vaporizer, a flow meter, and an induction chamber.
2.3 MRI Hardware
The general hardware requirements for renal 1H MRI on mice and rats are described in the chapter by Ramos Delgado P et al. “Hardware Considerations for Preclinical Magnetic Resonance of the Kidney.” The technique described in this chapter has been tailored for MR preclinical systems at magnetic fields higher than 3 T but advice for adaptation to other field strengths is given where necessary. No special or additional hardware is required, except for the following:
A physiological monitoring system that can track the respiration and which is connected to the MR system such that it can be used to trigger the MRI scanner with respiration. Typically, we use the MR-compatible rodent monitoring and gating system (Small Animal Instruments, New York, USA) equipped with an air-pillow to monitor breathing rate.
2.4 MRI Techniques
The diffusion MRI pulse sequence includes a diffusion preparation responsible for the diffusion sensitization. Specifically, sensitisation to diffusion and/or other motion of water molecules is achieved by inclusion of an extra pair of magnetic field gradients during the acquisition, generating images with varying degrees of diffusion-weighting. The degree of diffusion weighting depends on the area of each of the diffusion gradients (amplitude G and duration δ) as well as the time spacing between the pair (Δ); the resulting weighting is commonly specified by the compound parameter b-value (Fig. 1). The diffusion module is followed by fast image readout such as echo planar imaging (EPI). Other readouts are available for DWI (see Note 1), but DW-EPI is the standard since it provides the higher SNR in acceptable acquisition times, and so will be used in this chapter.
Diffusion preparation (“Diffusion gradients”). The degree of diffusion weighting (measured by the “b-value” [mm/s2]) depends on the area of the extra pair of gradients (amplitude G and duration δ) as well as the time spacing between them (Δ): \( b={\gamma}^2{G}^2{\delta}^2\left(\varDelta -\frac{\delta }{3}\right) \)
3 Methods
3.1 MR Protocol Setup
All the mentioned MR parameters are adjustable; the provided suggestions are based on experience, but the end protocol is likely to be adjusted from these values. For more details on what goes toward making these decisions please refer to the chapter by Jerome NP et al. “Renal Diffusion-Weighted Imaging (DWI) for Apparent Diffusion Coefficient (ADC), Intravoxel Incoherent Motion (IVIM), and Diffusion Tensor Imaging (DTI): Basic Concepts” of this book.
3.1.1 DW-EPI for ADC
-
1.
Load the DW-EPI sequence .
-
2.
Sequence type: 2D Echo Planar Imaging sequence with diffusion preparation module that has a defined Δ and δ and a G which can be incremented within a single scan. This is a standard sequence on Bruker MRI systems, called “DW-EPI.”
-
3.
The diffusion parameters are then adjusted to allow detection of incoherent movement of molecules. The range of displacements measured with DWI is typically in the order of 1–20 μm, allowing for quantitative measurements that reflect micromorphological and physiological changes in tissues. As a recommendation a δ = 3.5 ms and a Δ = 8.5 ms should be used (see Note 2).
-
4.
Set up your b-values to the following values: 0, 200, 600 s/mm2 over three orthogonal directions x: (1,0,0), y: (0,1,0) and z: (0,0,1).
-
5.
Repetition time (TR): choose ~500 ms for good signal stability and signal-to-noise per time (SNR/t) efficiency. TR will be limited by the length of the excitation pulse, length of echo train and the number of slices you acquire.
-
6.
Echo time (TE): use the shortest TE. Acquisition bandwidth should be considered to shorten the inter-echo-time.
-
7.
Segments: as low as possible to reduce scan time, however 1 segment (single-shot) might create unacceptable distortions on phase encoding. A high number of segments will increase scan time and makes the acquisition more prone to motion artifacts. As a recommendation two segments should be used (multishot DW-EPI) with a matrix size of 172 × 172 with an FOV of 30 × 30 mm.
-
8.
Acquisition bandwidth (BW): ~ 350.000 Hz, one wants to acquire as many lines of k-space as possible to minimize motion distortions of the images. For that reason, it is advantageous to have a low inter-echo-time. Larger inter-echo-times are not advisable because the SNR in the kidney will be so low that these images must be excluded.
-
9.
Enable fat saturation. On ultrahigh field systems this works well to avoid fat signal overlaying the kidney due to chemical shift. At lower field strengths it might work less efficient.
-
10.
Enable the respiration trigger (per slice). This is essential to reduce motion artefacts, reduce motion blurring and unwanted intensities variations among the images acquired with different b-values (see Note 3).
-
11.
Choose as phase-encoding direction the L-R direction and adapt the geometry so that the FOV in this direction includes the entire animal.
-
12.
Use frequency encoding in head-feet (rostral-caudal) direction to avoid severe aliasing. Adjust the FOV to your needs keeping in mind that in this direction the FOV can be smaller than the animal and a smaller FOV permits a smaller acquisition matrix, and in turn a shorter echo-spacing.
-
13.
Increase the number of averages to improve signal-to-noise ratio by a factor of √averages, especially important for higher b-values (use nine averages as a recommendation).
-
14.
To reach a steady-state magnetization is advisable to use dummy scans, two as a recommendation.
-
15.
For an example of a specific parameter set please see Notes 4 and 5.
-
16.
For adaptation of the geometry to rats (see Note 6).
3.1.2 DW-EPI for IVIM
-
1.
Load the DW-EPI sequence .
-
2.
Use the same parameters as DWI-EPI for ADC (TE, TR, matrix size, dummy scans, averages, bandwidth, segments).
-
3.
Keep the same diffusion parameters as for the ADC but increase the number of b-values from 3 to at least 7 (e.g., 0, 50, 100, 200, 400, 600, and 740 s/mm2) to probe fast diffusion from blood perfusion.
3.2 In Vivo DWI
3.2.1 Animal Preparation
-
1.
Anesthetize the animal and transfer it to scanner. For an in-depth description and discussion of the anesthesia please refer to the chapter by Kaucsar T et al. “Preparation and Monitoring of Small Animals in Renal MRI.”
-
2.
Start the temperature monitoring system, apply some surgical lubricant to the temperature probe and place it in the rectum of the animal.
-
3.
Attach the respiration sensor (e.g., balloon) to the chest of the animal using adhesive tape. Start and set up the respiratory monitoring system. If necessary, adjust the position of the respiration sensor until the amplitude of the respiration trace is sufficiently large for the system to reliably detect the trigger points at the beginning of expiration (see Note 3).
3.2.2 Scanner Adjustments and Anatomical Imaging
-
1.
Acquire a fast pilot scan to obtain images in three orthogonal planes x, y, and z.
-
2.
Acquire anatomical images in several oblique orientations to facilitate planning a coronal slice orientation with regard to the long axis of the kidney, as described in the chapter by Pohlmann A et al. “Essential Practical Steps for MRI of the Kidney in Experimental Research” (open-access).
-
3.
Perform localized shimming on the kidney as described in the chapter by Pohlmann A et al. “Essential Practical Steps for MRI of the Kidney in Experimental Research” (open-access). NB: shimming is crucial for DW-EPI (see Note 7), because a poor shim can lead to large distortions and add errors in the measured diffusion coefficients.
-
4.
Acquire a 3D B0 field-map without adaptation of the geometry (optional; see Notes 8 and 9).
3.2.3 Baseline Condition
-
1.
Load the 2D multishot EPI sequence , adapt the slice orientation to provide a coronal or axial view with respect to the kidney (in scanner coordinates this is double-oblique).
-
2.
In the monitoring unit set the trigger delay so that the trigger starts at the beginning of the expiratory plateau (no chest or diaphragm motion) and the duration such that it covers the entire expiratory phase, that is, until just before inhalation starts (1/2–2/3 of breath-to-breath interval) (see Note 3).
-
3.
In the monitoring unit set the trigger delay so that the trigger starts at the beginning of the expiratory plateau (no chest or diaphragm motion) and the duration to a short value, such as 10 ms (see Note 3).
-
4.
Run the EPI scan. Example images are shown in Fig. 2.
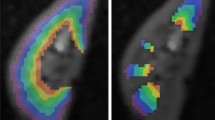
Series of six diffusion-weighted images of a healthy mouse kidney acquired with DW-EPI sequence at 7.0 T. Images correspond to b-values: 0, 50, 100, 200, 400, and 600 mm/s2. EPI readouts are prone to magnetic susceptibility artifacts, low-phase encoding bandwidth leads to ΔB0 induced frequency dispersions which causes image distortion. When using a segmented EPI readout a phase deviation occurs between the different segments in k-space leading to ghosting artefacts visible in the above images [7]
4 Notes
-
1.
Other readouts apart from EPI can be use in DWI such as rapid acquisition with relaxation enhancement (RARE) (Fig. 3) which is less prone to suffering from geometric distortion specially at ultrahigh magnetic fields [6].
-
2.
Gradients systems might differ from MR scanner to MR scanner even for the same magnetic field. If the gradient system cannot reach b-value of 600, consider to adjust δ = 3.5 ms and a Δ = 8.5 to higher values. Record and report all diffusion parameters used.
-
3.
You must monitor the respiration continuously throughout the entire experiment.
-
4.
Example for ADC of a 30 g mouse at 7 T (Bruker small animal system): TR = 500 ms; 3 b-values: 0, 200, 600 s/mm2, diffusion directions: 3 (orthogonal), effective TE = 56.87 ms; Segments = 2; averages = 9; slice thickness = 1.2 mm; slice orientation = axial; frequency encoding = head-feet; FOV = 30 × 30 mm; matrix size = 172 × 172; Bandwidth = 350.000 Hz; fat suppression = on; scan time ≈ 80 s.
-
5.
Example for ADC of a 270 g rat at 9.4 T (Bruker small animal system): TR = 500 ms; 3 b-values: 0, 200, 600 s/mm2, diffusion directions: 3 (orthogonal), effective TE = 56.87 ms; Segments = 2; averages = 9; slice thickness = 1.2 mm; slice orientation = axial; frequency encoding = head-feet; FOV = 45 × 45 mm; matrix size = 172 × 172; Bandwidth = 350.000 Hz; fat suppression = on;, scan time ≈ 80 s.
-
6.
For rats increase the FOV to the body width and keep the matrix size the same or similar. The relative resolution is then identical and the SNR should also be similar, because the larger rat RF coil provides worse SNR (e.g., eight-channel rat body phase array receive coil vs eight-channel mouse body phase array receive coil).
-
7.
Shimming is particularly important, since macroscopic magnetic field inhomogeneities affect EPI readout and might create severe geometric distortions. Shimming should be performed on a voxel enclosing the kidney using either the default iterative shimming method or the Mapshim technique (recommended).
-
8.
Example parameters for field mapping of a 30 g mice at 9.4 T (Bruker small animal system): use the vendors default protocol AnyObject > AnyRegion > Adjustments > ADJ_B0MAP. TR = 20 ms; flip angle = 30°; first echo = 1.60 ms; echo spacing = 3.57 ms; fat/water in-phase = on; slice orientation = main orientations (no angles) and offset = 0; FOC = (58 × 58 × 58) mm; matrix size =64 × 64 × 64; resolution = (0.904 × 0.904 × 0.904) mm; respiration trigger = off; acquisition time = 1–2 min.
-
9.
This serves to keep a record of the B0 influence on the measured DW-EPI images. It allows explanation of unusually distortions due to an imperfect shim.
Series of five diffusion-weighted images of a healthy mouse kidney acquired with single-shot DW-RARE sequence at 9.4 T. Images correspond to b-values: 0, 200, 300, 400, and 600 mm/s2. A RARE readout can be used to minimize the magnetic susceptibility artifacts which cause geometric distortions on DW-EPI. DW-RARE is not commercially available, pulse programming skills are needed to implement it [6]
References
Hahn EL (1950) Spin echoes. Phys Rev 80:580–594
Carr HY, Purcell EM (1954) Effects of diffusion on free precessions in nuclear magnetic resonance experiments. Phys Rev 94:630–635
Stejskal EO, Tanner JE (1965) Spin diffusion measurements: spin echoes in the presence of a time-dependent field gradient. J Chem Phys 42:288–292. https://doi.org/10.1063/1.1695690
Le Bihan D et al (1950) Imagerie de diffusion in-vivo par resonance magnetique. CR Acad Sci 15:1109–1112
Le Bihan D et al (1988) Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology 168(2):497–505
Periquito J et al (2020) Diffusion-weighted renal MRI at 9.4 Tesla using RARE to improve anatomical integrity. Scientific Reports 9(1):1–12
Paul K et al (2015) Diffusion-sensitized ophthalmic magnetic resonance imaging free of geometric distortion at 3.0 and 7.0 T: a feasibility study in healthy subjects and patients with intraocular masses. Investig Radiol 50:309–321
Acknowledgments
This work was funded, in part (Thoralf Niendorf, Andreas Pohlmann, and Joao Periquito), by the German Research Foundation (Gefoerdert durch die Deutsche Forschungsgemeinschaft (DFG), Projektnummer 394046635, SFB 1365, RENOPROTECTION. Funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation), Project number 394046635, SFB 1365, RENOPROTECTION).
This chapter is based upon work from COST Action PARENCHIMA, supported by European Cooperation in Science and Technology (COST). COST (www.cost.eu) is a funding agency for research and innovation networks. COST Actions help connect research initiatives across Europe and enable scientists to enrich their ideas by sharing them with their peers. This boosts their research, career, and innovation.
PARENCHIMA (renalmri.org) is a community-driven Action in the COST program of the European Union, which unites more than 200 experts in renal MRI from 30 countries with the aim to improve the reproducibility and standardization of renal MRI biomarkers.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Open Access This chapter is licensed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license and indicate if changes were made.
The images or other third party material in this chapter are included in the chapter's Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the chapter's Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
Copyright information
© 2021 The Author(s)
About this protocol
Cite this protocol
Periquito, J.S., Meier, M., Niendorf, T., Pohlmann, A., Jerome, N.P. (2021). Renal MRI Diffusion: Experimental Protocol. In: Pohlmann, A., Niendorf, T. (eds) Preclinical MRI of the Kidney. Methods in Molecular Biology, vol 2216. Humana, New York, NY. https://doi.org/10.1007/978-1-0716-0978-1_24
Download citation
DOI: https://doi.org/10.1007/978-1-0716-0978-1_24
Published:
Publisher Name: Humana, New York, NY
Print ISBN: 978-1-0716-0977-4
Online ISBN: 978-1-0716-0978-1
eBook Packages: Springer Protocols