Abstract
Background
Testicular torsion is an important pediatric problem and ischemia/reperfusion injury (IRI) is involved in its etiopathogenesis. Vanillic acid (VA) is a phenolic acid has strong antioxidant properties. To our knowledge, the ability of VA to reduce testicular IRI has not been previously investigated. It was therefore aimed to evaluate whether VA had a beneficial effect against testicular IRI model in rats for the first time. Twenty-four rats were segregated into four groups: sham control, torsion/detorsion (T/D), T/D + VA (50 mg/kg and 100 mg/kg). The levels of testicular oxidative stress, inflammation, endoplasmic reticulum (ER) stress and apoptosis markers were determined using colorimetric methods. Hematoxylin–eosin staining method was used in the histopathological evaluation.
Results
Oxidative stress, inflammation, ER stress and apoptosis levels were significantly higher in testicular tissues of rats with only IRI model (p < 0.05). VA applications improved these injuries in a dose-dependent manner (p < 0.05). Moreover, it was found that the results of histological examinations supported the biochemical results to a statistically significant extent.
Conclusions
It was revealed that VA application can remove testicular IRI for the first time. This testicular protective efficacy of VA needs to be supported by more extensive preclinical studies.
Similar content being viewed by others
1 Background
Testicular torsion (TT) causes occlusion of blood vessels and prevents perfusion of the testicles [1]. The annual incidence of TT is approximately 3.8 per 100,000 men younger than 18 years of age [2]. Mandatory treatment of TT is detorsion [1]. Transient ischemia caused by TT can permanently damage the testicles despite successful detorsion, and testicular apoptosis/atrophy and impaired spermatogenesis may develop [3]. Paradoxically, the production of free oxygen radicals, lipid peroxidation and intracellular calcium concentrations in the detorsioned testicle are increased and this situation causes more damage and may even initiate a process leading to cell death [1]. This is known as ischemia/reperfusion injury (IRI) and accepted main pathophysiological mechanism of torsion/detorsion (T/D)-induced testicular injury [2, 4].
Vanillic acid (VA) is one of the most scientifically researched phenolic compounds [5]. Previous studies have demonstrated the antioxidant, antimicrobial, anti-inflammatory, anti-apoptotic, anticancer, neuroprotective, hepatoprotective, renoprotective and cardioprotective properties of VA [6]. Although VA and its derivatives have been shown to protect heart, brain and liver against IRI in experimental models [7,8,9], there is no study examining the protection of VA against testicular IRI. This study therefore aimed to reveal protective efficacy of VA against testicular IRI model biochemically and histologically.
2 Methods
2.1 Experimental design
The experimental design was approved by the Animal Experiments Local Ethics Committee of Karadeniz Technical University (Protocol number: 2021/62). The 24 rats were divided into four groups: sham control (Group A), torsion/detorsion (T/D) (Group B), T/D + VA (50 and 100 mg/kg) (Group C and D). All drug and solvent applications were done by intraperitoneal route. The experimental procedure was briefly summarized in Table 1. In Group A rats, the left testicle was removed and placed back into the scrotum to create surgical stress. In Group B, C and D rats, the left testicle removed by incision was rotated 720° clockwise using the method described previously [10]. The suture was removed after 4 h, and testicular reperfusion was achieved for 2 h based on previously described methods [11, 12]. VA was administered 30 min before detorsion to the groups. VA doses (50 and 100 mg/kg) were determined considering previous studies [8, 13]. At the end of 360 min, all rats underwent left orchiectomy. The removed testicles were homogeneously divided longitudinally in two pieces, and one portion was frozen at − 80 °C for biochemical analysis and other parts were used for histological evaluation.
2.2 Histological analysis
Routine histological tissue follow-up was performed for testicular specimens fixed from Bouin’s solution. Prepared preparations were stained with H&E and evaluated under a light microscope [3]. Later, seminiferous tubule architecture and the levels of spermatogenesis were graded with the scoring system defined by Johnsen [14]. Scoring and histological evaluation were performed blindly by a histologist unaware of the groups.
2.3 Biochemical analysis
The tissue malondialdehyde (MDA) level was determined using a colorimetric method [15], while total oxidant status (TOS) (Cat No: RL0024) and total antioxidant status (TAS) (Cat No: RL0017) levels were determined using commercial colorimetric kits (Rel Assay Diagnostics, Gaziantep, Turkey). The oxidative stress index (OSI) levels were calculated with the following formula [12]:
The 8-hydroxy-2′-deoxyguanosine (8-OHdG, Cat No: EU2548), superoxide dismutase (SOD, Cat No: ER0332), catalase (CAT, Cat No: ER0264), high-mobility group box 1 (HMGB1, Cat No: ER0291), nuclear factor kappa B protein 65 (NF-κB p65, Cat No: ER1187), tumor necrosis factor-alpha (TNF-α, Cat No: ER1393), interleukin-6 (IL-6, Cat No: ER0042), myeloperoxidase (MPO, Cat No: ER0142), 78-kDa glucose-regulated protein (GRP78, Cat No: ER0562), activating transcription factor 6 (ATF6, Cat No: ER1645), C/EBP homologous protein (CHOP, Cat No: ER0694) and caspase-3 (Cat No: ER0143) levels in tissue samples were determined using enzyme-linked immunosorbent assay (ELISA) kits (Finetest, Wuhan, China) according to the manufacturer’s instructions.
2.4 Statistical analysis
All data were expressed as median and IQR. Statistical differences between groups were assessed by Kruskal–Wallis and followed by Mann–Whitney U test. p < 0.05 is considered statistically significant.
3 Results
3.1 Biochemical findings
All biochemical findings were presented in Table 2. The MDA, TOS, OSI and 8-OHdG levels of Group B were significantly elevated compared to Group A (all p = 0.004). The MDA, TOS, OSI and 8-OHdG levels of Group C were lower than Group B (p = 0.01, p = 0.037, p = 0.004 and p = 0.037, respectively). Similarly, VA (100 mg/kg) decreased levels of oxidative stress parameters compared with Group B (all p = 0.004).
The TAS, SOD and CAT levels were lower in Group B than Group A (all p = 0.004). The TAS and SOD levels were higher in Group C than Group B significantly (p = 0.004 and p = 0.02, respectively). However, VA (100 mg/kg) alleviated these levels significantly (all p = 0.004).
The HMGB1, NF-κB p65, TNF-α, IL-6 and MPO levels were higher in Group B than Group A significantly (all p = 0.004). The HMGB1, IL-6 and MPO levels were significantly lower in Group C than Group B (p = 0.01, p = 0.004 and p = 0.01, respectively). However, VA (100 mg/kg) alleviated these levels significantly (all p = 0.004).
The GRP78, ATF6, CHOP and caspase-3 levels were higher in Group B than Group A significantly (all p = 0.004). The GRP78, ATF6 and CHOP levels were lower in Group C than Group B (p = 0.037, p = 0.004 and p = 0.045, respectively). However, VA (100 mg/kg) alleviated these levels significantly (all p = 0.004). Interestingly, no significant differences were between Group A and Group D in terms of biochemical markers.
3.2 Histological findings
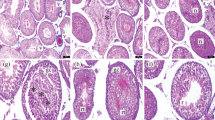
The Johnsen scores were lower in Group B than Group A significantly (p = 0.0001). Only VA (100 mg/kg) treatment increased these scores significantly (all p = 0.016) (Table 2 and Fig. 1).
Histopathological images of testicular tissues of groups (×200, H&E staining). Sham control group (A) SZ: spermatozoon, black star: seminiferous tubule germinal epithelium, black arrow: intertubular area. T/D group (B) VC: vasocongestion in the intertubular area, arrowhead: germinal epithelial cells, black star: seminiferous tubule germinal epithelium, black arrow: edema in the intertubular area. T/D + 50 mg/kg VA (C) SZ: spermatozoon, black star: seminiferous tubule germinal epithelium, arrowhead: germinal epithelial cells, black arrow: edema in the intertubular area. T/D + 100 mg/kg VA (D) SZ: spermatozoon, black star: seminiferous tubule germinal epithelium, black star: seminiferous tubule germinal epithelium, arrowhead: germinal epithelial cells, black arrow: intertubular area
4 Discussion
The pathogenesis of IRI is associated with complex molecular processes, including oxidative stress, ER stress and mitochondrial dysfunction [3, 11]. The increase in unbalanced reactive oxygen species (ROS) causes damage to biomolecules [9]. Peroxidation of lipids leads to the formation of aldehyde compounds, including MDA, which are highly reactive for all biomolecules [7]. TOS, TAS and OSI are cumulative oxidative stress indicators that reveal the level of oxidative stress in biological samples. 8-OHdG is an indicator of the extent of oxidative DNA damage [16]. SOD and CAT are two important components of the enzymatic antioxidant system that remove superoxide and H2O2, respectively, to prevent the formation of more reactive hydroxyl radicals. Therefore, these two antioxidant enzymes form the first line of cellular defense against oxidative damage [12]. The increased oxidative stress marker levels and decreased antioxidant capacity parameters in T/D group showing that oxidative stress mediates testicular IRI. VA treatments significantly reduced these damage. Consistently, it has been previously shown that VA and its derivatives can protect heart, brain and liver tissues in IRI models by reducing oxidative stress levels [7,8,9, 17].
It is known that inflammation is the another major pathway that plays critical role in the pathophysiology of T/D-induced testicular injury [18]. HMGB1 is a non-chromosomal nuclear protein that initiates the inflammatory process in pathological conditions, including IRI [19]. After IRI, extracellular HMGB1 operates as a damage-associated molecular pattern (DAMP) through two major signaling pathways. It interacts with TLR4 and RAGE receptors to activate NF-κB, which raises the levels of major cytokines, including TNF-α and IL-6 [19, 20]. The increased inflammatory marker levels in T/D group show that inflammation mediates testicular IRI. VA applications reduced the level of tissue inflammation. Consistently, it has been previously shown that VA can show protective activity against brain and cartilage IRI by reducing the levels of inflammation [8, 20].
Recent studies highlight that ER stress comes to the forefront as an important molecular mechanism in IRI-induced tissue damage [21]. ATF6 is one of major transmembrane proteins that initiate unfolded protein response (UPR) signaling in the ER stress response. Under physiological states, GRP78 binds to this sensor protein and keeps it in an inactive position [22]. ATF6 is activated by dissociation from GRP78 when ER stress is triggered. After ATF6 dissociates with GRP78, it is transported to Golgi in response to ER stress. ATF6 is activated via cleavaging by proteases in the Golgi. Activated ATF6 then migrates to the nucleus, inducing several proapoptotic genes, including CHOP [21]. CHOP activates the mitochondrial apoptosis pathway [22]. The results showed that increased ER stress and apoptosis contribute to testicular IRI, and VA attenuates this damage by ER stress inhibitory and anti-apoptotic effect. Consistently, it has been previously shown that VA and its derivatives exhibit tissue protective effect against various experimental IRI model through its ER stress inhibitor and anti-apoptotic properties [5, 23].
There were also some limitations of our study. First, only the two-stage doses of VA were evaluated based on literature data within the scope of this study. Second, the efficacy of VA at different times or in chronic T/D conditions was not evaluated. Third, the effects of VA on sexual behavior and fertility level of rats were not evaluated in the study. We believe that demonstrating the protective efficacy of VA against testicular IRI in long-term studies together with physiological fertility behavior experiments will shed light on clinical stages.
5 Conclusions
The results showed that VA reduced testicular IRI in an experimental model for the first time. This beneficial activity of VA is thought to be due to its antioxidant, anti-inflammatory, anti-apoptotic and ER stress inhibitor properties. The suitability of VA for clinical use needs to be supported by more detailed studies.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- 8-OHdG:
-
8-Hydroxy-2′-deoxyguanosine
- ATF6:
-
Activating transcription factor 6
- CAT:
-
Catalase
- CHOP:
-
C/EBP homologous protein
- DMSO:
-
Dimethyl sulfoxide
- ELISA:
-
Enzyme-linked immunosorbent assay
- ER:
-
Endoplasmic reticulum
- GRP78:
-
78-KDa glucose-regulated protein
- H&E:
-
Hematoxylin and eosin
- HMGB1:
-
High-mobility group box 1
- I/R:
-
Ischemia/reperfusion
- IL-6:
-
Interleukin-6
- IRI:
-
Ischemia/reperfusion injury
- IQR:
-
Interquartile range
- MDA:
-
Malondialdehyde
- MPO:
-
Myeloperoxidase
- NF-κB p65:
-
Nuclear factor kappa B protein 65
- OSI:
-
Oxidative stress index
- ROS:
-
Reactive oxygen species
- SOD:
-
Superoxide dismutase
- TAS:
-
Total antioxidant status
- T/D:
-
Torsion/detorsion
- TNF-α:
-
Tumor necrosis factor-alpha
- TOS:
-
Total oxidant status
- TT:
-
Testicular torsion
- UPR:
-
Unfolded protein response
- VA:
-
Vanillic acid
References
Shamsi-Gamchi N, Razi M, Behfar M (2021) Cross-link between mitochondrial-dependent apoptosis and cell cycle checkpoint proteins after experimental torsion and detorsion in rats. Gene 795:145793
Vaos G, Zavras N (2017) Antioxidants in experimental ischemia-reperfusion injury of the testis: where are we heading towards? World J Methodol 7(2):37–45
Kazaz IO, Demir S, Kerimoglu G, Colak F, Alemdar NT, Dogan SY et al (2022) Chlorogenic acid ameliorates torsion/detorsion-induced testicular injury via decreasing endoplasmic reticulum stress. J Pediatr Urol 18(3):289.e1-289.e7
Kohsaka T, Yoneda Y, Yoshida T, Minagawa I, Pitia AM, Iwasawa A et al (2022) Relaxin exerts a protective effect during ischemia-reperfusion in the rat model. Andrology 10(1):179–189
Punvittayagul C, Chariyakornkul A, Jarukamjorn K, Wongpoomchai R (2021) Protective role of vanillic acid against diethylnitrosamine- and 1,2-dimethylhydrazine-induced hepatocarcinogenesis in rats. Molecules 26(9):2718
Sharma N, Tiwari N, Vyas M, Khurana N, Muthuraman A, Utreja P (2020) An overview of therapeutic effects of vanillic acid. Plant Arch 20(Suppl 2):3053–3059
Dianat M, Hamzavi GR, Badavi M, Samarbafzadeh A (2014) Effects of losartan and vanillic acid co-administration on ischemia-reperfusion-induced oxidative stress in isolated rat heart. Iran Red Crescent Med J 16(7):e16664
Wang J, Guo Y, Zhang SY (2018) Vanillic acid improve neural function after focal cerebral ischemia-reperfusion rats. Int J Pharmacol 14:488–494
Ahn YH, Seok PR, Oh SJ, Choi JW, Shin JH (2019) A study on the protective effect of antioxidants on damage induced by liver ischemia/repefusion in a rat model. Korean J Clin Lab Sci 51(3):370–378
Turner TT, Tung KS, Tomomasa H, Wilson LW (1997) Acute testicular ischemia results in germ cell-specific apoptosis in the rat. Biol Reprod 57(6):1267–1274
Kazaz IO, Mentese A, Demir S, Kerimoglu G, Colak F, Bodur A et al (2020) Berberine inhibits the ischemia-reperfusion induced testicular injury through decreasing oxidative stress. Am J Emerg Med 38(1):33–37
Demir S, Kazaz IO, Kerimoglu G, Demir EA, Colak F, Yilmaz S et al (2022) Astaxanthin protects testicular tissue against torsion/detorsion-induced injury via suppressing endoplasmic reticulum stress in rats. J Invest Surg 35(5):1044–1049
Khoshnam SE, Sarkaki A, Khorsandi L, Winlow W, Badavi M, Moghaddam HF et al (2017) Vanillic acid attenuates effects of transient bilateral common carotid occlusion and reperfusion in rats. Biomed Pharmacother 96:667–674
Johnsen SG (1970) Testicular biopsy score count-A method for registration of spermatogenesis in human testes: normal values and results in 335 hypogonadal males. Hormones 1:2–25
Mihara M, Uchiyama M (1978) Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem 86(1):271–278
Demir EA, Mentese A, Kucuk H, Alemdar NT, Demir S (2022) p-Coumaric acid alleviates cisplatin-induced ovarian toxicity in rats. J Obstet Gynaecol Res 48(2):411–419
Lan XB, Wang Q, Yang JM, Ma L, Zhang WJ, Zheng P et al (2019) Neuroprotective effect of vanillin on hypoxic-ischemic brain damage in neonatal rats. Biomed Pharmacother 118:109196
Afolabi O, Alabi B, Omobowale T, Oluranti O, Iwalewa O (2021) Cysteamine mitigates torsion/detorsion-induced reperfusion injury via inhibition of apoptosis, oxidative stress and inflammatory responses in experimental rat model. Andrologia 54(1):e14243
Asavarut P, Zhao H, Gu J, Ma D (2013) The role of HMGB1 in inflammation-mediated organ injury. Acta Anaesthesiol Taiwan 51:28–33
Ziadlou R, Barbero A, Martin I, Wang X, Qin L, Alini M et al (2020) Anti-inflammatory and chondroprotective effects of vanillic acid and epimedin C in human osteoarthritic chondrocytes. Biomolecules 10(6):932
Han Y, Yuan M, Guo YS, Shen XY, Gao ZK, Bi X (2021) Mechanism of endoplasmic reticulum stress in cerebral ischemia. Front Cell Neurosci 15:704334
Chong WC, Shastri MD, Eri R (2017) Endoplasmic reticulum stress and oxidative stress: a vicious nexus implicated in bowel disease pathophysiology. Int J Mol Sci 18:771
Zhao J, Yang Y (2022) Vanillic acid alleviates lipopolysaccharides-induced endoplasmic reticulum stress and inflammation in human lung fibroblasts by inhibiting MAPK and NF-κB pathways. Qual Assur Saf Crops Foods 14(1):55–63
Acknowledgements
The authors wish to thank Sait Al and Ibrahim Aydin from Surgical Practice and Research Center of Karadeniz Technical University for professional assistance with the experimental studies.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
AM designed the study, methodology, formal analysis and writing—original draft. SD designed the study, methodology, formal analysis and writing—original draft. IOK designed the study, methodology and writing—original draft. EY designed the study, methodology and writing—original draft. NTA conducted the experimental protocols. EAD conducted the experimental protocols. MBK conducted the experimental protocols. TBD conducted the experimental protocols. YA designed the study and interpretation and revised the manuscript before submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Animal housing and handling were performed according to the recommendations of Experimental Animal Local Ethics Committee of Karadeniz Technical University with ethical approval had Protocol No: 2021/62 which followed the recommendations of the National Institutes of Health (NIH) Guide for Care and Use of Laboratory Animals (NIH Publication No. 8023, revised 1978).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mentese, A., Demir, S., Kazaz, I.O. et al. Vanillic acid attenuates testicular ischemia/reperfusion injury in rats. Beni-Suef Univ J Basic Appl Sci 11, 155 (2022). https://doi.org/10.1186/s43088-022-00336-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-022-00336-7