Abstract
Background
Adansonia digitata (L) fruit has a multi-purpose function one among many, is the antioxidant activities of the fruit by preventing oxidative stress. The effect of Adansonia digitata (L) fruit on lead-induced liver and kidney damage is not clear. Hence, the study was aimed to assessed the protective role of Adansonia digitata (L) fruits against lead acetate induced changes in the liver and kidney function test parameters and the histology of both organ in experimental rats. The rats were divided into five groups with five rats each. All the rats were administered with respective assigned treatment once daily for 6 weeks. Rats in groups I were administered with just distil water (2 ml/kg). Rats in groups II were administered with lead acetate (30 mg/kg) while rats in groups III–V were administered Adansonia digitatata (L) fruit extract (250 mg/kg and 500 mg/kg) and Succimer (5 mg/kg) respectively, then additionally challenged with lead acetate (30 mg/kg) immediately after. At the end of the administration, the blood serum from the experimental rats were used for biochemical analysis. Then, the the organs such as the liver and kidney collected for histological study.
Results
Rats administered with Lead acetate showed an increase in AST, ALP and ALT as well as increase in urea and creatinine level (p < 0.001), when compared with the control group (group I), where as Adansonia digitatata (L) fruit prevented the effect (upsurge of serum, Urea, Creatinine, AST, ALP and ALT) of lead acetate. Rats administer with only Lead acetate revealed marked liver steatosis and the degeneration of the kidney glomerulus. The Adansonia digitatata (L) fruit extract and Succimer prevented the histological liver steatosis, as well as the degeneration of the glomerulus of the kidney cytoarchitecture.
Conclusion
The findings in this study suggest that Adansonia digitata fruits extract has a protective potentials against lead acetate induced liver and kidney toxicity by preventing the upsurge of liver function enzymes and kidney function parameters. Hence, Adansonia digitata fruits can serve as a natural plant agent that can prevent hepato-renal toxicity. Therefore, Adansonia digitata holds future prospects in preclinical framework to ameliorate organs toxicity for oral therapeutic applications.
Similar content being viewed by others
1 Background
Lead (Pb) is a non-biodegradable toxic heavy metal found in the water, soil and air, it has a widespread contamination that result in serious health problems [1, 2]. Report from Institute for health and metric evaluation indicated that Pb toxicity result in about 9.3 million disabilities and 494,550 deaths globally [3]. Pb occur naturally in the environment, it constitutes a major environmental hazard globally [4]. The hazard and burden of Pb poisoning is high in developing countries. In Nigeria, high cases of Pb poisoning have been reported in parts Niger and Zamfara states [5]. Pb has been reported to cause multiple organ failure leading to disability and death. The toxic effect of Pb depends on the amount and period of exposure [6, 7]. Pb gets into the body by inhaling Pb containing substances or by ingesting Pb-contaminated food and/or water that are eventually absorbed into the blood stream via the respiratory and gastrointestinal tracts respectively [8]. In the body, lead binds to the red blood cells, distributed to different tissues and eventually accumulates in bone [9]. Diet can affect Pb absorption. Food rich in copper, iron zinc and calcium can decrease Pb uptake while food rich in fat and/or protein can promote Pb absorption [10]. Children are more prone to Pb poisoning because their organs are still in the developmental stage [11].
Adansonia digitata (Baobab) is a plant that is widely grown in the arid and semi-arid regions of Africa, America and Asia due to its ability to survive a long period of drought and high temperature [12, 13]. Boabab leaves and seed are used in making soup and as flavouring agent in many African dishes respectively while the fruit pulp is used for making beverages [14]. In Africa, baobab fruit is traditionally used in the treatment of dysentery and diarrhoea and to stimulate milk production in lactating women [15, 16]. Andansonia digitata fruit pulp is rich vitamin C, dietary fiber, calcium, potassium, procyanidins, kaempferol isomers, terpenoids, Flavoniods and thiamine [17, 18]. Baobab leaves and fruit are important sources of polyphenols, they are reported to have cardioprotective, antioxidant and hepatoprotective properties [19, 20]. The antioxidant property of baobab fruit is attributed to its vitamin C, polyphenols and flavonoids content [21]. With all the aforementioned beneficial effects of Adansonia digitata fruitpulp and leaves, the present study was aimed at evaluating the protective role of Adansonia digitata fruits pulp against lead induced liver and kidney injury in Rats.
2 Methods
2.1 Chemical
Lead acetate (BDH Chemicals Ltd, England) was used as hepato-renal toxicant. Succimer (meso-2,3 Dimercaptosuccinic acid) (Sigma-Aldrich, MO, USA) was used as standard drug (Chelating agent). Ketamine hydrochloride (PVT Ltd, India) was used as an anesthetic agent. Also, creatinine, urea, uric acid, AST, ALT and ALP kits (Randox test kits) were used.
2.2 Plant collection and authentication
Adensonia digitata (baobab) fruits were obtained from a local farm in Shika, Sabo Local Government, Kaduna state, Nigeria. The Fruit was authenticated at the Herbarium (specimen no. 2512) Department of Biological Sciences, Ahmadu Bello University (ABU), Zaria, Nigeria.
2.3 Extraction
The fruit pulp of Adansonia digitata was grounded and extracted with distilled water in a soxhlet apparatus for 10 h.
2.4 Experimental animals
A total of 25 albino Wistar rats (160–180 g) were purchased from the Department of Pharmacology, ABU Zaria. The rats were allowed to acclimatize to the animal house condition for two (2) weeks before the commencement of the study. The rats had free access to rat chow and water. The research was approved by Animal use and care committee (ABUCAUC/2018/064), Ahmadu Bello University Zaria, and conducted in accordance with the ARRIVE guidelines.
2.5 Experimental design
The rats were randomly distributed into five groups (n = 5). The grouping and administration of Lead acetate, succimer and aqueous extract of Adansonia digitata fruits is summarized in Table 1.
2.6 Biochemical studies
The rats were euthanized at the end of the six weeks, blood sample of each rat was collected in a plain tube. The blood samples were centrifuged at 3000 rmp for 5 min using a centrifuge machine. The serum obtained was used to estimate ALT, AST, ALP, creatinine, urea and uric acid.
2.7 Histological studies
The liver and kidneys were harvested and fixed in neutral buffered formalin, dehydrated in graded alcohol, embedded in paraffin wax, cleared in xylene, sectioned at 5 µm and stained with Hematoxylin and eosin (H & E).
2.8 Statistical analysis
Data were expressed as mean ± standard error (SE). One-way analysis of variance (ANOVA) test was followed by Tukey’s post-hoc test was conducted using Graphpad prsim (Version 9.2). Statistical significance was considered at p < 0.05.
3 Results
3.1 Liver function enzymes
The levels of ALP, AST and ALT increased significantly (p < 0.0001) in the serum of lead acetate control rats when compare to control rats and rats treated with Adansonia digitata and Succimer (Fig. 1). Treatment with 500 mg/kg of Adansonia digitata were shown to be more effective. There was no significant difference (p > 0.05) in AST level between control rats and rats treated with Adansonia digitata and Succimer (Fig. 1).
Bar chats of the liver enzymes parameters (ALP, AST and ALT) after 6 weeks of the experiment. Data were analysed using one way ANOVA, followed with Tukey post hoc test, *p < 0.033; **p < 0.002; ***p < 0.0001 for the experimental treated groups when compared with the control Group (DW). Data are mean ± SEM, n = 5 rats for each group
3.2 Kidney function parameters
A significant increase (p < 0.0001) in creatinine and urea levels were observed in the kidney serum of rats treated with only lead acetate when compared with the control rats and rats that were treated with Adansonia digitata and Succimer (Fig. 2). There was no significant difference (p > 0.05) in creatinine level between control rats and rats treated with Adansonia digitata and Succimer (Fig. 2).
Bar chats of the creatinine and Urea serum level after 6 weeks of the experiment. Data were analysed using one way ANOVA, followed with Tukey post hoc test, *p < 0.033; **p < 0.002; ***p < 0.0001 for the experimental treated groups when compared with the control Group (DW). Data are mean ± SEM, n = 5 rats for each group
3.3 Histological study
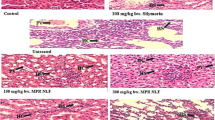
Photomicrograph of the liver of normal control rats showed normal hepatocytes central vein and sinusoids (Fig. 3a). The liver of rats treated with only lead acetate showed marked steatosis, fat hepatocellular vacuoles and degenerating hepatocytes (Fig. 3b). The liver of rats treated concomitantly with lead actetate (30 mg/kg) and Adansonia digitata (250 mg/kg and 500 mg/kg) showed mild steatosis (arrow) and some macro vesicular fatty change (Fig. 3c, d). The liver of rats treated with Lead acetate (30 mg/kg) and Succimer (5 mg/kg) showed mild macro vesicular fatty change (Fig. 3e). For the histology of the kidney, the kidney of normal control rats showed histological structure of the kidney with normal glomerulus and renal tubules (Fig. 4a). The kidney of rats treated with only Lead acetate (30 mg/kg) showed marked degenerated glomerulus and spontaneous lipid vacuolation of the glomerulus (Fig. 4b). The Kidney of rats treated with Lead acetate (30 mg/kg) and Adansonia digitata (250 mg/kg and 500 mg/kg) simultaneously showed normal glomerulus and some mild tubular damage (Fig. 4c, d). The kidney of rats treated with Lead acetate (30 mg/kg) and Succimer (5 mg/kg) showed mild degenerated glomerulus (star) marked tubular damage (Fig. 4e).
Photomicrographs of sections of liver of a control (a) showing normal histological structure of the liver with the central vein (star), Lead acetate treated micrograph (b) showing marked steatosis (arrow) and some macro vesicular fatty change (arrow head), Extract 250/500 mg/kg + lead acetate 30 mg/kg (c, d) respectively showing mild steatosis (arrow) and some macro vesicular fatty change (arrow head), Succimer 5 mg/kg + lead acetate 30 mg/kg micrograph (e) showing mild macro vesicular fatty change (arrow head). H&E stained X 250, n = 5
Photomicrographs of sections of kidney of a control (a) showing histological structure of the kidney with normal glomerulus (star) and renal tubules (arrow head), Lead acetate treated micrograph (b) showing marked degenerated glomerulus and spontaneous lipid vacuolation of the glomerulus (star), Extract 250/500 mg/kg + lead acetate 30 mg/kg (c, d) respectively showing normal glomerulus (star) and some mild tubular damage (arrow head), Succimer 5 mg/kg + lead acetate 30 mg/kg micrograph (e) showing mild degenerated glomerulus (star) marked tubular damage (arrow head). H&E stained X 250, n = 5
4 Discussion
In this present study, the hepatic and renal toxicity due to lead acetate was seen by a significant increase in the levels of parameters like ALT, AST and ALP as other studies also observed same outcome [22, 23]. This could be explained by the fact that Pb disrupt the liver cell membrane [24], hence the increased in concentration of the liver enzymes parameters. The marked increase in serum creatinine and urea level in lead acetate administered rats (Fig. 2), this could due to the toxic effect of lead acetate on the cells of renal tubules, hence the impairment of the permeability of creatinine and urea [25, 26]. the outcome in this experiment is consistent with that of previous studies [5].
Adansonia digitata fruit administered rats markedly protected the disruptions induced by lead acetate by reducing the level of the liver enzymes parameters (AST, ALP and ALT) likewise the serum level of urea and creatinine. This might be due to the antioxidant potential of Adansonia digitata fruit [21], which plays a significant role to protecting the liver and kidney against the lead acetate induced renal toxicity as reported in previous studies [27, 28]. The distortion induced by lead acetate on both tissue (liver and kidney) confirmed the biochemical parameters result and might be due the formation of free radicals generated by the lead acetate [25]. The histopathological findings of Rats administered with Adansonia digitata fruit reveal the restoration of cyto-architectural of the liver and kidney. The protective activities of Adansonia digitata fruit might be due to the fruit bioactive components, as it was reported that the fruits is rich in vitamin C, uroslic acids, and sitosterol [29], This components have been report to mediate against free radical, lipidemic and inflammatory activities [30, 31], hence, the hepatic and renal architectural protection by the Adansonia digitata fruit. The result is in agreement with previous studies [32].
5 Conclusions
This study suggested the protective role of Adansonia digitata fruit extract against lead acetate induced liver and kidney toxicity. Adansonia digitata fruit extract exerts this protective role by mitigating the upsurge of both liver function enzymes and kidney function parameters (creatininea and Urea) level. Likewise the study revealed the protective efficacy of the fruit extract against lead acetate induced hepatic and kidney cyto-architectural degeneration in rats.
Availability of data and Material
Not applicable.
Abbreviations
- AD:
-
Adansonia digitatat
- ALP:
-
Alkaline Phosphatase
- ALT:
-
Alanine Aminotransferase
- AST:
-
Aspartate Aminotransferase
- DW:
-
Distil Water (Normal Control group)
- Pb:
-
Lead
- SU:
-
Succimer (Standard drug group
References
Kalia K, Flora SJ (2005) Strategies for safe and effective therapeutic measures for chronic arsenic and lead poisoning. J Occup Health 47:1–21
Jarvis P, Quy K, Macadam J, Edwards M, Smith M (2018) Intake of lead (Pb) from tap water of homes with leaded and low lead plumbing systems. Sci Total Environ 644:1346–1356. https://doi.org/10.1016/j.scitotenv.2018.07.064
IHME (2017) Institute for health metrics and evaluation (IHME). University of Washington. GBD Comp, Seattle
Gillis BS, Arbieva Z, Gavin IM (2012) Analysis of lead toxicity in human cells. BMC Genom 13:344
Offor SJ, Mbagwu HOC, Orisakwe OE (2017) Lead induced hepato-renal damage in male albino rats and effects of activated charcoal. Front Pharmacol 8:107. https://doi.org/10.3389/fphar.2017.00107
Flora G, Gupta D, Tiwari A (2012) Toxicity of lead: a review with recent updates. Interdiscip Toxicol 5(2):47–58
Andjelkovic M, Djordjevic AB, Antonijevic E, Antonijevic B, Stanic M, Kotur-Stevuljevic J, Kalimanovska VS, Jovanovic M, Boricic N, Wallace D, Bulat Z (2019) Toxic effect of acute cadmium and lead exposure in rat blood, liver, and kidney. Int J Environ Res Public Health 16:274
Charkiewicz AE, Backstrand JR (2020) Lead toxicity and pollution in Poland. Int J Environ Res Public Health 17:4385
Mason LH, Harp JP, Han DY (2014) Pb neurotoxicity: neuropsychological effects of lead toxicity. BioMed Res Int. https://doi.org/10.1155/2014/840547
Wieczorek J, Baran A, Urbánski K, Mazurek R, Klimowicz-Pawlas A (2018) Assessment of the pollution and ecological risk of lead and cadmium in soils. Environ Geochem Health 40:2325–2342
Wani A, Ara A, Usmani JA (2015) Lead toxicity: a review. Interdiscip Toxicol 8(2):55–64. https://doi.org/10.1515/intox-2015-0009
Coe SA, Clegg M, Armengol M, Ryan L (2013) The polyphenol-rich baobab fruit (Adansonia digitata L.) reduces starch digestion and glycemic response in humans. Nutr Res 33:888–896
Rahul J, Jain MK, Singh SP, Kamal RK, Naz A, Gupta AK, Mrityunjay SK (2015) Adansonia digitata L. (baobab): a review of traditional information and taxonomic description. Asian Pac J Trop Biomed 5(1):79–84
Muthai KU, Karori MS, Muchugi A, Indieka AS, Dembele C, Mng’omba S, Jamnadass R (2017) Nutritional variation in baobab (Adansonia digitata L.) fruit pulp and seeds based on Africa geographical regions. Food Sci Nutr 5:1116–1129
Ibrahima C, Didier M, Max R, Pascal D, Benjamin Y, Renaud B (2013) Biochemical and nutritional properties of baobab pulp from endemic species of Madagascar and the African mainland. Afr J Agric Res 8(47):6046–6054
Li XN, Sun J, Shi H et al (2017) Profiling hydroxycinnamic acid glycosides, iridoid glycosides, and phenylethanoid glycosides in baobab fruit pulp (Adansonia digitata). Food Res Int 99:755–761
Simbo DJ, De Smedt S, Van den Bilcke N, De Meulenaer B, Van Cam J, Uytterhoeven V, Samson R (2013) Opportunities for domesticating the African baobab (Adansonia digitata L.): multi-trait fruit selection. Agrofor Syst 87(3):493–505
Braca A, Sinisgalli C, De Leo M, Muscatello B, Cioni P, Milella L, Ostuni A, Giani S, Sanogo R (2018) Phytochemical profile, antioxidant and antidiabetic activities of Adansonia digitata L. (Baobab) from Mali, as a source of health-promoting compounds. Molecules 23:3104. https://doi.org/10.3390/molecules23123104
Ghoneim MAM, Hassan AI, Mahmoud MG, Asker MS (2016) Protective effect of Adansonia digitata against isoproterenol-induced myocardial injury in rats. Anim Biotechnol 27(2):84–95. https://doi.org/10.1080/10495398.2015.1102147
Tembo DT, Holmes MJ, Marshall LJ (2017) Effect of thermal treatment and storage on bioactive compounds, organic acids and antioxidant activity of baobab fruit (Adansonia digitata) pulp from Malawi. J Food Compos Anal 58:40–51. https://doi.org/10.1016/j.jfca.2017.01.002
Sokeng AJT, Sobolevb AP, Lorenzo AD, Xiao J, Luisa M, Donatella C, Maria D (2019) Metabolite characterization of powdered fruits and leaves from Adansonia digitata L. (baobab): a multi-methodological approach. Food Chem 272(2019):93–108
Ibrahim NM, Eweis EA, El-Beltagi HS, Abdel-Mobdy YE (2012) Effect of lead acetate toxicity on experimental male albino rat. Asian Pac J Trop Biomed 20(1):41–46
Abdel-Moneim AM, El-Toweissy MY, Ali AM (2015) Curcumin ameliorates lead induced hemato-biochemical alterations and renal oxidative damage in a rat model. Biol Trace Elem Res 168:206–220
Amin I, Hussain I, Rehman MU, Mir BA, Ganaie SA, Ahmad SB, Rahman MU, Shanaz S, Muzamil S, Arafah A, Ahmad P (2020) Zingerone prevents lead-induced toxicity in liver and kidney tissues by regulating the oxidative damage in Wistar rats. J Food Biochem. https://doi.org/10.1111/jfbc.13241
Ekong EB, Jaar BG, Weaver VM (2006) Lead-related nephrotoxicity: a review of the epidemiologic evidence. Kidney Int 70(12):2074–2084
Yuan G, Dai S, Yin Z (2014) Sub-chronic lead and cadmium co-induce apoptosis protein expression in liver and kidney of rats. Int J Clin Exp Pathol 7(6):2905
Hanafy A, Aldawsari HM, Badr JM, Ibrahim AK, Abdel-Hady SE (2016) Evaluation of hepatoprotective activity of Adansonia digitata extract on acetaminophen-induced hepatotoxicity in rats. Evid Based Complement Altern Med. https://doi.org/10.1155/2016/4579149
Ntchapda F, Bonabe C, Atsamo AD, Azambou DRK, Fouda YB, Djibrine SI, SekeEtet PF, Théophile D (2020) Effect of aqueous extract of Adansonia digitata stem bark on the development of hypertension in L-NAME-induced hypertensive rat model. Evid Based Complement Altern Med 69:10. https://doi.org/10.1155/2020/3678469
Kaboré H, Sawadogo-Lingani B, Diawara C, Compaoré M, Dicko MJ (2011) A review of baobab (Adansonia digitata) products: effect of processing techniques, medicinal properties and uses. Afr J Food Sci 5:833
Oliveira EA, Chaves MH, Almeida FRC, Lima RCP, Silva RM, Maia JL et al (2005) Protective effect of alpha- and beta-amyrin, a triterpene mixture from Protium heptaphyllum (Aubl.) March. Trunk wood resin, against acetaminopheninduced liver injury in mice. J Ethnopharmacol 98(1–2):103–108
Santos EA, Frota JT, Arruda BR, de Melo TS, Ade A, da Silva CA, Ade G, Brito C et al (2012) Antihyperglycemic and hypolipidemic effects of α, β-amyrin, a triterpenoid mixture from Protium heptaphyllum in mice. Lipids Health Dis 6:98
Suliman HM, Osman B, Abdoon IH, Saad AM, Khalid H (2020) Ameliorative activity of Adansonia digitata fruit on high sugar/high fat dietsimulated metabolic syndrome model in male Wistar rats. Biomed Pharmacother 125:109968
Acknowledgements
None.
Funding
The authors declare no specific funding for this work.
Author information
Authors and Affiliations
Contributions
Conception and design: All authors. Provision of study materials: WM, ESO and NID. Data collection: WM, ESO and SAB. Data analysis and interpretation: All authors; Initial draft of manuscript: WM, BI, SAB and NID. Critical review of the manuscript: WM and NID. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The research was approved by ABU Directorate of Academic Planning and Monitoring (Approval No: ABUCAUC/2018/064) and was conducted according to the ARRIVE Guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Makena, W., Otong, E.S., Dibal, N.I. et al. Aqueous fruit pulp extract of Adansonia digitata (L) protects against lead-acetate-induced hepato-renal damage in rat model. Beni-Suef Univ J Basic Appl Sci 10, 59 (2021). https://doi.org/10.1186/s43088-021-00151-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43088-021-00151-6