Abstract
Background
In spite of the huge advances in recent medicine, there is no effective drug that completely protects the liver from toxic materials. This study was conducted to investigate the hepatoprotective effect of arctigenin from burdock (Arctium lappa) against carbon tetrachloride (CCl4)-induced liver injury.
Results
Arctigenin pre-administration reduced hepatotoxicity markers significantly as compared to CCl4 group. In addition, both silymarin and arctigenin declined matrix metalloproteinase-2 (MMP-2) in the serum (1177 ± 176), (978 ± 135) significantly as compared to CCl4 group (1734 ± 294). The hepatic antioxidant parameters (total glutathione, superoxide dismutase, and glutathione reductase) were significantly decreased after CCl4 injection, an effect that has been prevented by pre-administration of both silymarin and arctigenin. Histological examinations illustrated that arctigenin reduced CCl4 damage, where it decreased inflammation, congestion, and ballooning.
Conclusions
Arctigenin exerted a hepatoprotective effect against CCl4-induced liver damage in terms of suppressing MMP-2 and oxidative stress comparative to that of silymarin.
Similar content being viewed by others
Background
An organ as complex as the liver can be susceptible to a variety of problems. However, in an unhealthy or malfunctioning liver, the outcomes can be dangerous or even fatal. Liver cirrhosis is one of liver serious problems; it is a frequent consequence of the long clinical course of all chronic liver diseases and is characterized by tissue fibrosis and the conversion of normal liver architecture into structurally abnormal nodules [1, 2].
Liver diseases were the 10th leading cause of death for men and the 12th for women in the USA, killing about 27,000 people each year. Also, the cost of liver diseases in terms of human suffering, hospital costs, and lost productivity is very high [3].
The extracellular matrix (ECM), formed by the complex network of proteins and sugars surrounding cells in all solid tissues, is among the most important regulators of cellular and tissue functions in the body [4]. In addition to providing structural support for cells, ECM regulates various cellular functions, such as adhesion, migration, differentiation, proliferation, and survival. Cellular responses are context-dependent, and dysregulation of ECM production and proteolysis is often associated with the development of liver pathology [5]. Matrix metalloproteinases (MMPs) are a family of over 24 zinc-dependent endopeptidases capable of degrading virtually any component of the ECM. MMPs have emerged as essential mediators in defining how cells interact with their surrounding microenvironment in normal liver [6].
Many plants have important roles in human health care. There are some plants that are consumed habitually by humans and that have been proven as hepatoprotective capacity, for example, artichoke [7,8,9], milk thistle [10, 11], grapefruit, and chamomile [12, 13].
Arctigenin (AG) is an aglycon of arctiin [14]. It is a bioactive lignan isolated from the seeds of burdock (Arctium lappa) [15], acts as an antioxidant. The phenolic content of burdock is antioxidant too [16, 17]. As previous studies confirmed that Arctium lappa extract has hepatoprotective effect [18], there is no survey related to AG alone (which is one of the constituents of Arctium lappa), has the same effect, knowing that AG has an antioxidant [19], anti-inflammatory [20], and gastroprotective properties [21]. This study was aimed to investigate the hepatoprotective effect of AG on CCl4-induced liver toxicity in experimental rats, in terms of hepatic markers, MMP-2, oxidative stress, and histopathological changes.
Methods
This study was conducted in the Experimental Animal Laboratory of the Faculty of Pharmacy, Al-Ahliyya Amman University, and ethically approved by ethical committee for the care and use of laboratory animals (ethical approval no. AAU-1/14/2017-2018).
Chemicals, reagents, and kits
Arctigenin (Item No. 270652), glutathione (GSH) kit (Item No.703002), superoxide dismutase (SOD) kit (Item No. 706002), and glutathione reductase kit (Item No. 703202) were purchased from (Cayman chemicals, USA). CCl4 (> 99.9%, Item No. 270652), carboxymethylcellulose (CMC) (Item No. C9481), and metphosphoric acid (MPA) (Item No. 79613) from Sigma Aldrich, USA.
Total MMP-2 ELISA kit (Item No. MMP200) and lysis buffer (Item No. 895347) were supplied by RnD Systems, USA. Alanine aminotransferase (ALT) (Item No. 11533), aspartate aminotransferase (AST) (Item No. 11531), alkaline phosphatase (ALP) (Item No.11592), and bilirubin (Item No. 11515) assay kits were purchased from BioSystems S.A., Barcelona (Spain). Silymarin (Legalon® 70 mg) was kindly provided by Chemical Industries Development CID, Egypt.
Animals
A total of 24 male Wistar rats (age 6-8 weeks, weight 210-240 g) were provided from the Jordanian University of Science and Technology (JUST), Irbid, Jordan. All animals were kept under observation in Al-Ahliyya Amman University animal house, for 2 weeks prior to the study with free access to commercial rat diet and water ad libitum. Rats were housed at 22 ± 2 °C with a 12 h light-dark cycle. All animals’ handling and treatment were in adherence to the ARRIVE guidelines.
Experimental design
The rats were randomly divided into 4 equal groups (n = 6 rats). Group A (control) and B (toxic), animals were administered the vehicle daily (1% CMC, 4 mL/kg, i.p.). Group C (standard), rats were daily administered silymarin (200 mg/kg, 4 mL/kg, i.p.) [22]. Group D (treatment), rats were daily administered AG (15 mg/kg, 4 mL/kg, i.p.) [19] (Fig. 1). All animals were treated for 6 weeks. The experimental design was approved by the ethical committee in Al-Ahliyya Amman University.
Induction of hepatotoxicity
A single dose of CCl4 (1 mL/kg, i.p.) was chosen according to Kandil et al. [23]. Diluted CCl4 solution was prepared by dissolving CCl4 in olive oil (1:1) to prevent its evaporation. On the last day of the designated period, animals were overnight fasted before the injection with diluted CCl4 (groups of B, C, and D) or olive oil (2 mL/kg, group A). One hour later, they were provided with food. On the next day, animals were fasted for 4 h, lightly anesthetized then sacrificed by cervical dislocation after taking the blood samples.
Blood samples
Twenty-four hours after CCl4 injection, blood samples were withdrawn by heparinized capillary tubes from a retro-orbital vein under light anesthesia using a piece of cotton immersed in diethyl-ether [24], allowed to clot for 30 min, sera were separated by centrifugation at RCF 1000×g for 10 min. Four aliquots were prepared from each serum and stored at −20 °C until analysis.
Liver tissue specimens
After blood samples collection, animals were sacrificed by cervical dislocation, livers were taken using histological scissors, rinsed with cold saline, dried on a filter paper, and photographed. A portion of each liver was excised, put in 10% formalin solution, and processed as for routine histological evaluation. The remaining part of each liver was stored at −80 °C for later oxidative stress analyses [23].
Liver tissue homogenization
Around 50 mg sample was excised from each liver and homogenized in 1 ml of cell lysis buffer using Teflon homogenizer in ice. The lysate was then cold-centrifuged at RCF 10,000×g for 15 min at 4 °C. Supernatants were distributed into four Eppendorf tubes and stored at −80 °C to be analyzed later.
Histological investigation
Five-micrometer sections were stained with hematoxylin-eosin, examined using a light microscope (Leica). and photographed using MC 170 HD Leica Camera (Switzerland) and LAS EZ software. The histological sections were investigated by 2 of the authors in a blinded fashion.
Serum parameters
Serum ALT, AST, ALP, total bilirubin, and total MMP-2 were analyzed 24 h after induction of hepatotoxicity according to manufacturer instructions.
Oxidative stress
Hepatic total protein was determined in the tissue homogenate according to Lowry method [25], total GSH was assayed according to Eyer et al. [26] method. Briefly, the clear supernatant obtained from the homogenate was first deproteinized using 5% MPA then Ellman’s reagent (5,5′-dithiobis-2-nitrobenzoic acid) was added which is reduced by sulfhydryl group of GSH to yield a yellow color with a maximum absorbance at 405-412 nm. The concentration was expressed as nM/mg tissue.
Superoxide dismutase activity was analyzed according to Spits and Oberley [27] utilizing a tetrazolium salt for the detection of superoxide radicals generated by xanthine oxidase and hypoxanthine. One unit of SOD is defined as the amount of enzyme needed to exhibit 50% dismutation of the superoxide radical.
Glutathione reductase activity was measured by measuring the rate of NADPH oxidation which is accompanied by a decrease in absorbance at 340 nm [28].
Statistical analyses
All descriptive statistics, analyses, and graphics were performed using GraphPad Prism version 6 (GraphPad Software, San Diego. USA). Data passed the Shapiro-Wilk normality test and were expressed in tables as mean, standard deviation, and standard error of the mean. One-way analysis of variance (ANOVA) followed by Tukey-Kramer post-analysis procedure was used to compare the means of all groups. Differences between means were considered statistically significant at P ≤ 0.05.
Results
Hepatotoxicity markers
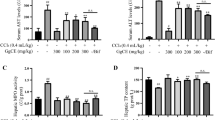
As shown in Table 1, a single injection of CCl4 significantly increased all hepatotoxicity markers as compared to control group, an effect that was inhibited by pre-administration of both silymarin and AG.
Serum total MMP-2
Serum total MMP-2 (ng/ml) was significantly higher (1734 ± 294) in CCl4 group than the control group. Both silymarin and AG maintained the level of MMP-2 close to the control group (1177 ± 176), (978 ± 135), (844 ± 178), respectively (Fig. 2).
Hepatic oxidative stress markers (Fig. 3)
Hepatic total GSH level (nM/mg tissue) after CCl4 injection was significantly lower (0.75 ± 0.20) as compared to the control group (1.26 ± 0.07). On the contrary, both silymarin (1.08 ± 0.13) and AG (1.18 ± 0.13) showed higher GSH levels in the hepatic tissues. However, AG and silymarin GSH levels were close to each other and lied between those of control and CCl4.
Hepatic SOD activity (U/mg protein) of CCl4 group was diminished significantly (1.65 ± 0.48) when compared to the control group (3.60 ± 0.25). AG injected-group showed SOD activity (2.34 ± 0.17) higher than that of CCl4 but lower than the control group. Also, silymarin pretreatment induced SOD activity (2.71 ± 0.38).
Single CCl4 injection lowered the glutathione reductase activity in the hepatic tissues (2.90 ± 1.35) significantly as compared to the control group (6.78 ± 1.55); this effect of CCl4 was prevented by the administration of both silymarin (5.49 ± 1.46) and AG (4.59 ± 0.78).
Histopathological results
Figure 4a shows a section in control liver tissue which demonstrates normal histology; central vein and each lobule are bordered by the “portal triad” consisting of a (branch of the hepatic artery, portal vein, and bile duct), in addition to hepatocytes surrounding it which are in sinusoids.
Ballooning (degeneration of hepatocytes) is obvious in CCl4-injected group section (Fig. 4b). Cells are pale (lightly stained); parenchymal cells show necrotic and apoptotic alterations, in addition to an increase in the number of inflammatory cells (WBCs), and congestion (RBCs).
Figure 4c shows that silymarin repaired some of the damage which is caused by CCl4 when they were injected respectively. Silymarin group section shows less inflammation and less ballooning. AG also protected the liver tissue from CCl4 damage (Fig. 4d); it is obvious that ballooning, congestion, and inflammation were reduced.
Discussion
Due to its large size, exclusive structure, and essential roles in maintaining homeostasis, the liver is subjected to many types of diseases and toxic agents [1]. CCl4 is commonly used for free radical-induced liver injury as many experimental and clinical studies consider it as a classical hepatotoxic agent that induces liver cirrhosis, fibrosis, and necrosis [29]. People may easily be exposed to it by inhalation or skin absorption due to its various usage such as in fire extinguisher and in refrigerant gas [30].
It is now generally accepted that CCl4 toxicity results from bioactivation of CCl4 into trichloromethyl free radical by cytochrome P450 system in liver microsomes and consequently causes lipid peroxidation of membranes that leads to liver damage. The free radicals generated by CCl4 metabolism attack polyunsaturated fatty acids in cell membranes forming (fatty acid) free radicals and induce lipid peroxidation with the production of reactive aldehydes, which lead to oxidative stress [31]. Lipid peroxidation causes cell membrane disruption leading to increased cell membrane permeability and enzyme leakage. This, in turn, activates cellular proteases, phospholipid, and protein degradation leading to cytotoxicity and inflammatory response [32]. Antioxidants and anti-inflammatory agents play a critical role against CCl4 intoxication by scavenging active oxygen and free radicals and neutralizing lipid peroxides [33].
We have used CCl4 rat model to investigate the hepatoprotective effect of AG in terms of hepatotoxic markers, antioxidant activities, MMP-2, and histopathological outcomes. Male Wistar rats were the animals of choice due to their higher ability to withstand CCl4-induced hepatotoxicity and to avoid any hormonal changes that may interfere with study outcomes when use females [23]. Silymarin (200 mg/kg) was used as a standard drug due to its reported hepatoprotective benefits [34].
Inflamed or injured hepatocytes leak high amounts of its contents including enzymes into the bloodstream; these enzymes are perfect indicators for diagnosis of hepatocellular damage [35].
After injection of CCl4, hepatotoxicity parameters ALT, AST, ALP, and bilirubin, were significantly increased as compared to the control group. Results that are supported by many previous studies [36, 37]. In our study, previous silymarin and AG administration demonstrated decreased levels in ALT, AST, ALP, and bilirubin. The same results were obtained by Lee et al. and Talwar et al. [37, 38] when they tested silymarin efficacy and found that it suppressed CCl4 damage and normalized hepatotoxicity markers due to its free radical scavenging effect.
According to oxidative stress parameters, there was a significant drop in GSH, SOD, and glutathione reductase in the liver of CCl4-injected animals compared to the control group. This effect agrees with Abdel-Moneim et al.’s study, when they investigated CCl4 efficacy on rat models too [36]. On the other hand, pretreatment with AG or silymarin expressed higher levels in these main liver antioxidant parameters. GSH, SOD, and glutathione reductase were normalized by silymarin which could diminish oxidative stress produced by ethanol gavage in mice. It was able to enhance mitochondrial metabolic processes and electron transport chain, to increase intracellular SOD activity, which led to the drop of intracellular ROS levels to improve mitochondrial function [34].
Matrix metalloproteinase-2 is also an important indicator of liver impairment. Our results elucidated the upregulation of this enzyme in rats injected with CCl4. Liang et al. proved this effect after the application of CCl4 in rats too, owing that to the activation of hepatic stellate cells (HSCs) by CCl4 which in turn increases MMP-2 expression. This enzyme increase resulted in hepatocytes matrix degradation, which ends in basement membrane destruction and initiating inflammatory cells to recruit to the injured site [39]. Feher and Lengyel found that silymarin has a beneficial effect on liver carcinogenesis explained by attenuating MMP-2 which is involved in invasion and angiogenesis [40].
When AG and silymarin were injected into rats in the current study, MMP-2 level was declined; findings that are supported by Kara et al. where silymarin lowered total MMP-2 activity knowing that total MMP-2 activity increases in hepatic decay [41]. In addition, Clichici et al. reported that administration of silymarin reduced inflammatory mediators including MMP-9 and liver fibrosis [42].
Conclusion
In conclusion, this study emphasized that AG played an obvious protective role from the harmful effect of CCl4 on rats’ liver. In addition to that, AG protective efficacy with a dose of 15 mg/kg/day, is very close to that of silymarin 200 mg/kg in the term of hepatotoxicity markers, oxidative stress parameters, MMP-2, and histopathological observations.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- AG:
-
Arctigenin
- ALP:
-
Alkaline phosphatase
- ALT:
-
Alanine aminotransferase
- ANOVA:
-
Analysis of variance
- AST:
-
Aspartate aminotransferase
- CCl4 :
-
Carbon tetrachloride
- CMC:
-
Carboxymethylcellulose
- ECM:
-
Extracellular matrix
- GSH:
-
Glutathione
- HSCs:
-
Hepatic stellate cells
- JUST:
-
Jordan University of Science and Technology
- MMP:
-
Matrix metalloproteinase
- MPA:
-
Metphosphoric acid
- NADP+ :
-
Nicotinamide adenine dinucleotide phosphate
- RBCs:
-
Red blood corpuscles
- RCF:
-
Relative centrifugal force
- ROS:
-
Reactive oxygen species
- SD:
-
Standard deviation
- SE:
-
Standard error
- SOD:
-
Superoxide dismutase
- WBCs:
-
White blood cells
References
Tarantino G, Citro V, Capone D (2019) Nonalcoholic fatty liver disease: a challenge from mechanisms to therapy. J Clin Med 9(1). https://doi.org/10.3390/jcm9010015
Zeng F, Zhang Y, Han X, Weng J, Gao Y (2019) Liver buds and liver organoids: new tools for liver development, disease and medical application. Stem Cell Rev Rep 15(6):774–784. https://doi.org/10.1007/s12015-019-09909-z
Anushiravani A, Ghajarieh Sepanlou S (2019) Burden of liver diseases: a review from Iran. Middle East. J Dig Dis 11(4):189–191 10.15171/mejdd.2019.147
Li L, Zhao Q, Kong W (2018) Extracellular matrix remodeling and cardiac fibrosis. Matrix Biol 68-69:490–506. https://doi.org/10.1016/j.matbio.2018.01.013
Friedman SL, Maher JJ, Bissell DM (2000) Mechanisms and therapy of hepatic fibrosis: report of the. AASLD Single Topic Basic Res Conf 32(6):1403–1408. https://doi.org/10.1053/jhep.2000.20243
Hamada T, Fondevila C, Busuttil RW, Coito AJ (2008) Metalloproteinase-9 deficiency protects against hepatic ischemia/reperfusion injury. Hepatology (Baltimore, Md) 47(1):186–198. https://doi.org/10.1002/hep.21922
Elsayed Elgarawany G, Abdou AG, Maher Taie D, Motawea SM (2019) Hepatoprotective effect of artichoke leaf extracts in comparison with silymarin on acetaminophen-induced hepatotoxicity in mice. J Immunoass Immunochem:1–13. https://doi.org/10.1080/15321819.2019.1692029
Panahi Y, Kianpour P, Mohtashami R, Atkin SL, Butler AE, Jafari R, Badeli R, Sahebkar A (2018) Efficacy of artichoke leaf extract in non-alcoholic fatty liver disease: a pilot double-blind randomized controlled trial. Phytotherapy research : PTR 32(7):1382–1387. https://doi.org/10.1002/ptr.6073
Tang X, Wei R, Deng A, Lei T (2017) Protective effects of ethanolic extracts from artichoke, an edible herbal medicine, against acute alcohol-induced liver injury in mice. Nutrients 9(9). https://doi.org/10.3390/nu9091000
Jung HA, Abdul QA, Byun JS, Joung EJ, Gwon WG, Lee MS, Kim HR, Choi JS (2017) Protective effects of flavonoids isolated from Korean milk thistle Cirsium japonicum var. maackii (Maxim.) Matsum on tert-butyl hydroperoxide-induced hepatotoxicity in HepG2 cells. J Ethnopharmacol 209:62–72. https://doi.org/10.1016/j.jep.2017.07.027
Abenavoli L, Izzo AA, Milic N, Cicala C, Santini A, Capasso R (2018) Milk thistle (Silybum marianum): a concise overview on its chemistry, pharmacological, and nutraceutical uses in liver diseases. Phytotherapy research : PTR 32(11):2202–2213. https://doi.org/10.1002/ptr.6171
Ebada ME (2018) Essential oils of green cumin and chamomile partially protect against acute acetaminophen hepatotoxicity in rats. An Acad Bras Cienc 90(2 suppl 1):2347–2358. https://doi.org/10.1590/0001-3765201820170825
Madrigal-Santillán E, Madrigal-Bujaidar E, Álvarez-González I, Sumaya-Martínez MT, Gutiérrez-Salinas J, Bautista M, Morales-González Á, MG-L YG-R, Aguilar-Faisal JL, Morales-González JA (2014) Review of natural products with hepatoprotective effects. World J Gastroenterol 20(40):14787
Hayashi K, Narutaki K, Nagaoka Y, Hayashi T, Uesato S (2010) Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biol Pharm Bull 33(7):1199–1205. https://doi.org/10.1248/bpb.33.1199
Lou C, Zhu Z, Zhao Y, Zhu R, Zhao H (2017) Arctigenin, a lignan from Arctium lappa L., inhibits metastasis of human breast cancer cells through the downregulation of MMP-2/-9 and heparanase in MDA-MB-231 cells. Oncol Rep 37(1):179–184. https://doi.org/10.3892/or.2016.5269
Ferracane R, Graziani G, Gallo M, Fogliano V, Ritieni A (2010) Metabolic profile of the bioactive compounds of burdock (Arctium lappa) seeds, roots and leaves. J Pharm Biomed Anal 51(2):399–404. https://doi.org/10.1016/j.jpba.2009.03.018
Romualdo GR, Silva EDA, Da Silva TC, Aloia TPA, Nogueira MS, De Castro IA, Vinken M, Barbisan LF, Cogliati B (2019) Burdock (Arctium lappa L.) root attenuates preneoplastic lesion development in a diet and thioacetamide-induced model of steatohepatitis-associated hepatocarcinogenesis. Environ Toxicol. https://doi.org/10.1002/tox.22887
Lin SC, Lin CH, Lin CC, Lin YH, Chen CF, Chen IC, Wang LY (2002) Hepatoprotective effects of Arctium lappa Linne on liver injuries induced by chronic ethanol consumption and potentiated by carbon tetrachloride. J Biomed Sci 9(5):401–409. https://doi.org/10.1007/BF02256533
Ming Wu R, Yan Sun Y, Ting Zhou T, Yuan Zhu Z, Jing Zhuang J, Tang X, Chen J, Hong Hu L, Shen X (2014) Arctigenin enhances swimming endurance of sedentary rats partially by regulation of antioxidant pathways. Acta Pharmacol Sin 35(10):1274–1284. https://doi.org/10.1038/aps.2014.70
Kang HS, Lee JY, Kim CJ (2008) Anti-inflammatory activity of arctigenin from Forsythiae Fructus. J Ethnopharmacol 116(2):305–312. https://doi.org/10.1016/j.jep.2007.11.030
Li XM, Miao Y, Su QY, Yao JC, Li HH, Zhang GM (2016) Gastroprotective effects of arctigenin of Arctium lappa L. on a rat model of gastric ulcers. Biomed Rep. https://doi.org/10.3892/br.2016.770
Navidi-Shishaone M, Mohhebi S, Nematbakhsh M, Roozbehani S, Talebi A, Pezeshki Z, Eshraghi-Jazi F, Mazaheri S, Shirdavani S, Gharagozloo M, Moaeidi BA (2014) Co-administration of silymarin and deferoxamine against kidney, liver and heart iron deposition in male iron overload rat model. Int J Prev Med 5(1):110–116
Kandil YI, Maraqa AD, Oriquat GA, Shraideh ZA (2017) Resveratrol pretreatment reduces circulating inflammatory interleukins in CCl4-induced hepatotoxicity rats. Bull Faculty Pharmacy Cairo Univ 55(2):319–323. https://doi.org/10.1016/j.bfopcu.2017.09.005
Parasuraman S, Raveendran R, Kesavan R (2010) Blood sample collection in small laboratory animals. J Pharmacol Pharmacother 1(2):87–93. https://doi.org/10.4103/0976-500X.72350
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193(1):265–275. https://doi.org/10.1016/0304-3894(92)87011-4
Eyer P, Podhradsky D (1986) Evaluation of the micromethod for determination of glutathione using enzymatic cycling and Ellman’s reagent. Anal Biochem 153(1):57–66. https://doi.org/10.1016/0003-2697(86)90061-8
Spitz DR, Oberley LW (1989) An assay for superoxide dismutase activity in mammalian tissue homogenates. Anal Biochem 179(1):8–18. https://doi.org/10.1016/0003-2697(89)90192-9
Mannervik B (2001) Measurement of glutathione reductase activity. Curr Protoc Toxicol Chapter 7:Unit7 2. https://doi.org/10.1002/0471140856.tx0702s00
Liu H, Zhang Z, Hu H, Zhang C, Niu M, Li R, Wang J, Bai Z, Xiao X (2018) Protective effects of Liuweiwuling tablets on carbon tetrachloride-induced hepatic fibrosis in rats. BMC Complement Altern Med 18(1):212. https://doi.org/10.1186/s12906-018-2276-8
Yehye WA, Rahman NA, Ariffin A, Abd Hamid SB, Alhadi AA, Kadir FA, Yaeghoobi M (2015) Understanding the chemistry behind the antioxidant activities of butylated hydroxytoluene (BHT): a review. Eur J Med Chem 101:295–312. https://doi.org/10.1016/j.ejmech.2015.06.026
Manibusan MK, Odin M, Eastmond DA (2007) Postulated carbon tetrachloride mode of action: a review. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 25(3):185–209. https://doi.org/10.1080/10590500701569398
Kim DH, Kwack SJ, Yoon KS, Choi JS, Lee BM (2015) 4-Hydroxynonenal: a superior oxidative biomarker compared to malondialdehyde and carbonyl content induced by carbon tetrachloride in rats. J Toxicol Environ Health A 78(16):1051–1062. https://doi.org/10.1080/15287394.2015.1067505
Ohta Y, Ohashi K, Matsura T, Tokunaga K, Kitagawa A, Yamada K (2008) Octacosanol attenuates disrupted hepatic reactive oxygen species metabolism associated with acute liver injury progression in rats intoxicated with carbon tetrachloride. J Clin Biochem Nutr 42(2):118–125. https://doi.org/10.3164/jcbn.2008017
Federico A, Dallio M, Loguercio C (2017) Silymarin/silybin and chronic liver disease: a marriage of many years. Molecules 22(2). https://doi.org/10.3390/molecules22020191
Limdi JK, Hyde GM (2003) Evaluation of abnormal liver function tests. Postgrad Med J 79(932):307–312. https://doi.org/10.1136/pmj.79.932.307
Abdel-Moneim AM, Al-Kahtani MA, El-Kersh MA, Al-Omair MA (2015) Free radical-scavenging, anti-inflammatory/anti-fibrotic and hepatoprotective actions of taurine and silymarin against CCl4 induced rat liver damage. PLoS One 10(12):e0144509. https://doi.org/10.1371/journal.pone.0144509
Lee CP, Shih PH, Hsu CL, Yen GC (2007) Hepatoprotection of tea seed oil (Camellia oleifera Abel.) against CCl4-induced oxidative damage in rats. Food Chem Toxicol 45(6):888–895. https://doi.org/10.1016/j.fct.2006.11.007
Talwar S, Jagani HV, Nayak PG, Kumar N, Kishore A, Bansal P, Shenoy RR, Nandakumar K (2013) Toxicological evaluation of Terminalia paniculata bark extract and its protective effect against CCl4-induced liver injury in rodents. BMC Complement Altern Med 13:127. https://doi.org/10.1186/1472-6882-13-127
Liang B, Guo XL, Jin J, Ma YC, Feng ZQ (2015) Glycyrrhizic acid inhibits apoptosis and fibrosis in carbon-tetrachloride-induced rat liver injury. World J Gastroenterol 21(17):5271–5280. https://doi.org/10.3748/wjg.v21.i17.5271
Feher J, Lengyel G (2012) Silymarin in the prevention and treatment of liver diseases and primary liver cancer. Curr Pharm Biotechnol 13(1):210–217. https://doi.org/10.2174/138920112798868818
Kara E, Coşkun T, Kaya Y, Yumuş O, Vatansever S, Var A (2008) Effects of silymarin and pentoxifylline on matrix metalloproteinase-1 and-2 expression and apoptosis in experimental hepatic fibrosis. Curr Ther Res 69(6):488–502
Clichici S, Olteanu D, Filip A, Nagy AL, Oros A, Mircea PA (2016) Beneficial effects of silymarin after the discontinuation of CCl4-induced liver fibrosis. J Med Food 19(8):789–797. https://doi.org/10.1089/jmf.2015.0104
Acknowledgements
This work was conducted with the support of Al-Ahliyya Amman University.
Funding
No fund was supplied to this study.
Author information
Authors and Affiliations
Contributions
GK performed the analytical methods. IA contributed in the proposal and manuscript writing. YK was a major contributor in performing the analytical methods, collecting and analyzing data, and writing the manuscript. All authors have read and approved the manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This study was ethically approved by ethical committee for the care and use of laboratory animals at Al-Ahliyya Amman University (ethical approval no. AAU-1/14/2017-2018).
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kanawati, G.M., Al-Khateeb, I.H. & Kandil, Y.I. Arctigenin attenuates CCl4-induced hepatotoxicity through suppressing matrix metalloproteinase-2 and oxidative stress. Egypt Liver Journal 11, 1 (2021). https://doi.org/10.1186/s43066-020-00072-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43066-020-00072-6