Abstract
Background
Chest computed tomography (CT) findings provide great added value in characterizing the extent of disease and severity of pulmonary involvements. Chest CT severity score (CT-SS) could be considered as an appropriate prognostic factor for mortality prediction in patients with COVID-19 pneumonia. In this study, we performed a meta-analysis evaluating the prognostic accuracy of CT-SS for mortality prediction in patients with COVID-19 pneumonia.
Methods
A systematic search was conducted on Web of Science, PubMed, Embase, Scopus, and Google Scholar databases between December 2019 and September 2021. The meta-analysis was performed using the random-effects model, and sensitivity and specificity (with 95%CIs) of CT-SS were calculated using the study authors’ pre-specified threshold.
Results
Sensitivity estimates ranged from 0.32 to 1.00, and the pooled estimate of sensitivity was 0.67 [95%CI (0.59–0.75)]. Specificity estimates ranged from 0.53 to 0.95 and the pooled estimate of specificity was 0.79 [95%CI (0.74–0.84)]. Results of meta-regression analysis showed that radiologist experiences did not affect the sensitivity and specificity of CT-SS to predict mortality in COVID-19 patients (P = 0.314 and 0.283, respectively). The test for subgroup differences suggests that study location significantly modifies sensitivity and specificity of CT-SS to predict mortality in COVID-19 patients. The area under the summary receiver operator characteristic (ROC) curve was 0.8248.
Conclusion
Our results have shown that CT-SS has acceptable prognostic accuracy for mortality prediction in COVID-19 patients. This simple scoring method could help to improve the management of high-risk patients with COVID-19.
Similar content being viewed by others
Introduction
In December 2019, coronavirus disease 2019 (COVID-19) emerged from Wuhan, China [1, 2]. This novel coronavirus disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was highly transmissible. The basic reproductive ratio (R0) of SARS-CoV-2 ranges from 2.2 to 3.9 [3, 4]. Therefore, COVID-19 spread rapidly throughout the world and the World Health Organization (WHO) declared it a pandemic on March 11, 2020 [5]. From December 2019 to October 8, 2021, a total of 237,809,466 confirmed cases and 4,853,001 deaths due to COVID-19 had been reported across the world [6]. Due to the high mortality rate of COVID-19, identification of the potential prognostic factors associated with the fatal outcomes would play a critical role to predict different orders of risk for COVID-19 patients. Early identification of patients at higher risk of death would help to improve patient management and better allocation of medical resources.
Studies have focused on determining different prognostic factors such as laboratory tests, comorbidities, and radiological manifestations [7,8,9,10,11,12,13]. Computed tomography (CT) as the most common method used in the diagnosis of COVID-19 has a high sensitivity to depict pulmonary pneumonia [14]. Chest CT findings provide great added value in characterizing the extent of disease and severity of pulmonary involvements [15]. This approach reports the extent of pulmonary involvement in chest CT severity score (CT-SS), which would help in clinical decision-making for symptomatic patients or even those without clinical symptoms. In retrospective case series, investigators have shown that the extent of lung damage was more pronounced in deceased patients as compared to survivors [16]. Therefore, CT-SS could be considered as an appropriate prognostic factor for mortality prediction in patients with COVID-19 pneumonia.
However, while several case series have shown a significant correlation between CT-SS and mortality of the COVID-19 patients, there are no systematic review and meta-analysis reporting the prognostic accuracy of CT-SS for mortality prediction in COVID-19 patients. In this study, we performed a meta-analysis evaluating the prognostic accuracy of CT-SS for mortality prediction in patients with COVID-19 pneumonia.
Methods
Protocol of the systematic review and meta-analysis
This systematic review and meta-analysis was performed following a pre-defined protocol and reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist [17].
Information sources and search strategies
Web of Science, PubMed, Embase, Scopus, and Google Scholar were searched to find studies reporting prognostic accuracy of CT-SS for mortality prediction in patients with COVID-19 pneumonia between December 2019 and September 2021. The review was conducted using the following keywords and logical operators: ((covid-19 OR SARS-CoV-2 OR 2019 Novel Coronavirus OR 2019-nCoV OR Wuhan virus OR severe acute respiratory syndrome coronavirus 2 OR coronavirus disease 2019) AND (CT OR computed tomography OR Chest computed tomography OR Chest CT OR X-Ray CT Scan OR X-Ray CAT Scan OR CT scan OR CAT scan) AND (Mortality OR Death OR decease* OR died OR dead)). The bibliographic lists of included articles were also reviewed.
Literature screening and assessment of the studies for inclusion were independently performed by two reviewers (NN and DR). Any disagreement was resolved by consulting a third investigator (SSZ).
The original studies investigating the prognostic performance of CT-SS for mortality prediction in COVID-19 patients were eligible to be included. Studies with unavailable full texts and insufficient data to calculate sensitivity and specificity were excluded.
Study selection and data collection process
Two reviewers (NN and DR) independently extracted principal study characteristics from the included studies. The first author of the selected articles, publication year, country, mean age of patients, sensitivity, and specificity (with 95%CIs) of CT-SS for mortality prediction in COVID-19 patients, sample size, and the gender ratio of males were extracted. The extracted data were checked by the third author (SSZ), and any disagreement between the authors was resolved through discussion. The characteristics of included studies are presented in Table 1.
Summary measures and synthesis of results
Data synthesis was conducted using Stata version 11.0 (Stata Corporation, College Station, TX, USA) and Review Manager 5.3 software (The Nordic Cochrane Centre, The Cochrane Collaboration). For each study, sensitivity and specificity (with 95% CIs) of CT-SS for mortality prediction in COVID-19 patients were calculated using the study authors’ pre-specified threshold.
Heterogeneity between studies was assessed using Cochran’s Q test and the Inconsistency index (I2) test (heterogeneity was defined as P < 0.1). For further assessment of the possible sources of between-study heterogeneity, the meta-regression and subgroup analysis were applied. The test for subgroup differences was performed according to study locations to evaluate whether study locations affects the sensitivity and specificity of CT-SS to predict mortality in COVID-19 patients. For meta-regression analysis, the moderator variable was the radiologist experience and it was plotted against sensitivity and specificity of CT-SS. Visual inspection of the generated funnel plot was employed to evaluate publication bias among studies. The summary ROC curve was also generated to evaluate the overall prognostic performance of CT-SS for mortality prediction in COVID-19 patients.
Results
Study selection
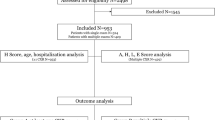
The literature search process is detailed in Fig. 1. The search strategy retrieved 7463 studies. Of the 3302 records after removing duplicate records, 25 articles were selected for further assessment based on the title and abstract. After the full-text assessment of the remaining studies, one study was excluded because it did not provide sufficient data to calculate sensitivity and specificity. Twenty-four studies were eventually included in the meta-analytical processes.
Study characteristics
The characteristics of the included studies are presented in Table 1. The first author, publication year, country, mean age of patients, sample size, and the gender ratio of males have been presented in the table. For each study, the sensitivity and specificity (with 95%CIs) of CT-SS were also listed in the table.
Risk of bias within studies
The P-values obtained from the Chi-squared test of heterogeneity were < 0.001 for sensitivity and specificity of CT-SS. Moreover, the results of I2 test for sensitivity and specificity of CT-SS were calculated as 87.8% and 92.5%, respectively. Therefore, the random-effects model of the meta-analysis was applied for evaluating the prognostic accuracy of CT-SS for mortality prediction in patients with COVID-19 pneumonia.
Synthesis of results
The forest plots of sensitivities and specificities of CT-SS for mortality prediction in COVID-19 patients are presented in Fig. 2a, b, respectively. Sensitivity estimates ranged from 0.32 to 1.00, and the pooled estimate of sensitivity was 0.67 [95% CI (0.59–0.75)] (Fig. 2a). Specificity estimates ranged from 0.53 to 0.95, and the pooled estimate of specificity was 0.79 [95% CI (0.74–0.84)] (Fig. 2b).
Forest plot for 24 included studies. a In this plot, pooled data evaluating the sensitivity of CT-SS to predict mortality in COVID-19 patients have been demonstrated under the random-effects model. The pooled estimate for sensitivity was calculated as 0.67 (95% CI, 0.59–0.75). b In this plot, pooled data evaluating the specificity of CT-SS to predict mortality in COVID-19 patients have been demonstrated under the random-effects model. The pooled estimate for specificity was calculated as 0.79 (95% CI, 0.74–0.84)
Risk of bias across studies
In Fig. 3, the funnel plots were considered to be moderately asymmetrical in shape which demonstrates the existence of publication bias in the results of included studies.
Funnel plots for 24 included studies. Visual inspection of the generated funnel plots was employed to evaluate publication bias among studies. The funnel plots appear asymmetrical. a In this plot, the X and Y axes represent sensitivity and standard errors, respectively. b In this plot, the X and Y axes represent specificity and standard errors, respectively
The P-values obtained in meta-regression analysis to evaluate the effect of the radiologist experiences on the sensitivity and specificity of CT-SS were P = 0.314 and 0.283, respectively (analysis not presented).
The sensitivity and specificity of CT-SS for mortality prediction of COVID-19 patients were categorized and subgrouped according to study locations. The overall effect size for each subgroup was calculated and is shown in Fig. 4.
Forest plot for 24 included studies according to the location of studies. a In this presentation, pooled data evaluating the sensitivity of CT-SS to predict mortality in COVID-19 patients have been demonstrated for each country under the random-effects model. b In this presentation, pooled data evaluating the specificity of CT-SS to predict mortality in COVID-19 patients have been demonstrated for each country under the random-effects model
The corresponding summary ROC plot is shown in Fig. 5. The area under the summary ROC curve was 0.8248.
Discussion
Severe or critical patients with COVID-19 are of great concern. They mostly have a poor prognosis and high mortality [40, 41]. Effective predictive models for early identification of patients at higher risk of death would improve patient management and help clinicians decide what intensity of care each patient needs. In the last two years, chest imaging combined with clinical evaluation and laboratory tests had played an essential role in patient management. In addition to symptomatic patients, radiographic lung injury abnormalities could even be manifested in asymptomatic cases. In Xie et al. study, chest CT findings of patients with COVID-19 infection who had initial negative reverse-transcriptase polymerase chain reaction results were reported. Repeated swab tests eventually confirmed COVID-19 infection of the patients [4]. The diagnostic value of radiological manifestations is already recognized for COVID-19.
Although CT is now a common method in the diagnosis of COVID-19, there is a lack of clinical evidence about its prognostic role for mortality prediction in COVID-19 patients. Deceased patients had higher CT-SSs versus those who recovered [16] which suggests that severe radiological manifestations may indicate a poor prognosis. In this study, we investigated the prognostic accuracy of CT-SS for mortality prediction in COVID-19 patients by conducting a meta-analysis. To date, no meta-analysis studies have been conducted on this aspect. To our knowledge, this report is the first meta-analysis describing the prognostic accuracy of CT-SS for mortality prediction in COVID-19 patients.
The retrieved studies have investigated deceased and survival patients with COVID-19 pneumonia, which allows an assessment of the overall CT performance in terms of sensitivity and specificity. All studies are retrospective and researchers had known the outcomes of patients. There is no potential risk of bias regarding the index test because a pre-specified diagnostic threshold was used for CT interpretations.
The results of our meta-analysis showed that CT-SS has, respectively, achieved pooled sensitivity and specificity values of 67% (95% CI, 59–75%) and 79% (95% CI, 74– 84%) in mortality prediction of COVID-19 patients. The classifiers have inherent strengths and limitations. There are two reasons to observe false negative results in radiological manifestations of COVID-19 patients. First, symptomatic patients may not have pulmonary involvement in the early course of the disease [41, 42]. Second, pneumonia may not develop in symptomatic upper respiratory tract infections [43, 44]. On the other hand, patient infection with other viral types of pneumonia such as various forms of flu could also result in false positive cases [45].
Radiologist experience is one of the effective factors which may have affected the predictive performance of CT-SS [46]. Results of meta-regression analysis showed that radiologist experiences did not affect the sensitivity and specificity of CT-SS to predict mortality in COVID-19 patients (P = 0.314 and 0.283, respectively). The test for subgroup differences based on study location suggests that there is a statistically significant subgroup effect for sensitivity and specificity of CT-SS (P = 0.01 and < 0.01, respectively), meaning that study location significantly modifies sensitivity and specificity of CT-SS to predict mortality in COVID-19 patients. These differences can be due to the heterogeneity in the severity of illness and epidemic [46]. There are also differences in the experience of treatment staff, health care, and hospital equipment in studied countries which may cause different mortality rates.
In summary, in ROC curve analysis, a considerable area under the curve was achieved for CT-SS. Therefore, CT-SS has acceptable performance for mortality prediction in COVID-19 patients. A simple and rapid approach with high sensitivity, which results in a low number of false negatives, is the preferred method for mortality prediction of COVID-19 patients. Such a system could help to improve the management of high-risk patients with COVID-19 even when they are clinically silent. From the results, it could be concluded that CT-SS has acceptable prognostic accuracy for mortality prediction in COVID-19 patients. This simple scoring method could help for triage of patients and screening of the patients with a higher need for intensive care.
Despite the significant prognostic value of the CT-SS, some limitations should be acknowledged for this parameter. There is a wide scoring range from 20 to 40 regions in the reported scores which makes assessments more difficult. Therefore, this parameter is inherently complex and time-consuming for clinical use. Second, the right lung is larger than the left one. So, their corresponding lobes and segments have different sizes. Dedicated software and an experienced specialist are required to consider these differences in semiquantitative and quantitative studies [47].
Conclusion
In this study, the prognostic accuracy of CT-SS for mortality prediction in COVID-19 patients was investigated. Our results have shown that CT-SS has acceptable prognostic accuracy for mortality prediction in COVID-19 patients. This simple scoring method could help to improve the management of high-risk patients with COVID-19.
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- CT-SS:
-
Chest CT severity score
- CT:
-
Computed tomography
- CI:
-
Confidence interval
- Summary ROC:
-
Summary receiver operator characteristic curve
References
Li Q, Guan X, Wu P et al (2020) Early transmission dynamics in Wuhan, China, of novel coronavirus–infected pneumonia. N Engl J Med 382:1199–1207
Lai CC, Shih TP, Ko WC, Tang HJ, Hsueh PR (2020) Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and coronavirus disease-2019 (COVID-19): The epidemic and the challenges. Int J Antimicrob Agents 55:1–9
Hilton J, Keeling MJ (2020) Estimation of country-level basic reproductive ratios for novel Coronavirus (SARS-CoV-2/COVID-19) using synthetic contact matrices. PLoS Comput Biol 16:1–10
Mair MD, Hussain M, Siddiqui S et al (2021) A systematic review and meta-analysis comparing the diagnostic accuracy of initial RT-PCR and CT scan in suspected COVID-19 patients. Br J Radiol 94:1–8
Santura I, Kawalec P, Furman M, Bochenek T (2021) Chest computed tomography versus RT-PCR in early diagnostics of COVID-19–a systematic review with meta-analysis. Pol J Radiol 86:518–531
Worldometer COVID-19 Data. https://www.worldometers.info/coronavirus/?zarsrc=130. Accessed 8 October 2021.
Tahtasakal CA, Oncul A, Sevgi DY et al (2021) Could we predict the prognosis of the COVID-19 disease? J Med Virol 93:2420–2430
Wang L, He W, Yu X et al (2020) Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect 80:639–645
Wang F, Hou H, Wang T et al (2020) Establishing a model for predicting the outcome of COVID-19 based on combination of laboratory tests. Travel Med Infect Dis 36:1–7
Pérez FM, Del Pino JL, García NJ et al (2020) Comorbidity and prognostic factors on admission in a COVID-19 cohort of a general hospital. Rev Clin Esp. https://doi.org/10.1016/j.rceng.2020.05.010
Cheng A, Hu L, Wang Y et al (2020) Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int J Antimicrob Agents 56:1–7
Hu Y, Zhan C, Chen C, Ai T, Xia L (2020) Chest CT findings related to mortality of patients with COVID-19: a retrospective case-series study. PLoS ONE 15:1–12
Yuan M, Yin W, Tao Z, Tan W, Hu Y (2020) Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE 15:1–10
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Muller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722
Angeli E, Dalto S, Marchese S et al (2021) Prognostic value of CT integrated with clinical and laboratory data during the first peak of the COVID-19 pandemic in Northern Italy: a nomogram to predict unfavorable outcome. Eur J Radiol 137:1–10
Lei Q, Li G, Ma X et al (2021) Correlation between CT findings and outcomes in 46 patients with coronavirus disease 2019. Sci Rep 11:1–6
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:1–34
Abdollahi I, Nabahati M, Javanian M, Shirafkan H, Mehraeen R (2021) Can initial chest CT scan predict status and clinical outcomes of COVID-19 infection? A retrospective cohort study. Egypt J Radiol Nucl Med 52:1–10
Bayrak V, Durukan NŞ, Aydemir FD et al (2021) Risk factors associated with mortality in ıntensive care COVID-19 patients: the importance of chest CT score and intubation timing as risk factors. Turk J Med Sci 51:1665–1674
Besutti G, Ottone M, Fasano T et al (2021) The value of computed tomography in assessing the risk of death in COVID-19 patients presenting to the emergency room. Eur Radiol. https://doi.org/10.1007/s00330-021-07993-9
Charpentier E, Soulat G, Fayol A et al (2021) Visual lung damage CT score at hospital admission of COVID-19 patients and 30-day mortality. Eur Radiol. https://doi.org/10.1007/s00330-021-07938-2
Dilek O, Demirel E, Akkaya H, Belibagli MC, Soker G, Gulek B (2021) Different chest CT scoring systems in patients with COVID-19: could baseline CT be a helpful tool in predicting survival in patients with matched ages and co-morbid conditions? Acta Radiol. https://doi.org/10.1177/02841851211006316
Guillo E, Gomez IB, Dangeard S et al (2020) COVID-19 pneumonia: diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur J Radiol 131:1–5
Hajiahmadi S, Shayganfar A, Janghorbani M et al (2021) Chest computed tomography severity score to predict adverse outcomes of patients with COVID-19. Infect Chemother 53:308–318
Isik SA, Samanci C, Yesildal M, Ekinci SC, Sarikaya B, Yenilmez E (2021) Relationship of radiological stage with laboratory parameters and mortality in covid-19 pneumonia. Acta Med Mediterr 37:445–451. https://doi.org/10.19193/0393-6384_2021_1_69
Kazemi MA, Ghanaati H, Moradi B et al (2020) Prognostic factors of initial chest ct findings for icu admission and mortality in patients with covid-19 pneumonia. Iran J Radiol 17:1–10
Kimura-Sandoval Y, Arévalo-Molina ME, Cristancho-Rojas CN et al (2021) Validation of chest computed tomography artificial intelligence to determine the requirement for mechanical ventilation and risk of mortality in hospitalized coronavirus disease-19 Patients in a tertiary care center in Mexico City. Rev Investig Clin 73:111–119
Li H, Luo S, Zhang Y, Xiao X, Liu H (2021) Longitudinal chest CT features in severe/critical COVID-19 cases and the predictive value of the initial CT for mortality. J Inflamm Res 14:1111–1124
Li K, Chen D, Chen S et al (2020) Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res 21:1–10
Li K, Wu J, Wu F et al (2020) The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Investig Radiol 55:1–5
Li Y, Yang Z, Ai T, Wu S, Xia L (2020) Association of “initial CT” findings with mortality in older patients with coronavirus disease 2019 (COVID-19). Eur Radiol 30:6186–6193
Magdy AM, Saad MA, El Khateeb AF, Ahmed MI, El-Din DHG (2021) Comparative evaluation of semi-quantitative CT-severity scoring versus serum lactate dehydrogenase as prognostic biomarkers for disease severity and clinical outcome of COVID-19 patients. Egypt J Radiol Nucl Med 52:1–11
Mirza-Aghazadeh-Attari M, Zarrintan A, Nezami N et al (2020) Predictors of coronavirus disease 19 (COVID-19) pneumonitis outcome based on computed tomography (CT) imaging obtained prior to hospitalization: a retrospective study. Emerg Radiol 27:653–661
Raoufi M, Naini SAAS, Azizan Z et al (2020) Correlation between chest computed tomography scan findings and mortality of COVID-19 cases; a cross sectional study. Arch Acad Emerg Med 8:1–7. https://doi.org/10.22037/aaem.v8i1.719
Ruch Y, Kaeuffer C, Ohana M et al (2020) CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect 26:5–8
Salahshour F, Mehrabinejad M-M, Toosi MN et al (2021) Clinical and chest CT features as a predictive tool for COVID-19 clinical progress: introducing a novel semi-quantitative scoring system. Eur Radiol. https://doi.org/10.1007/s00330-020-07623-w
Salvatore C, Roberta F, Angela dL, et al (2021) Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol Med 126:29–39
Tabatabaei SMH, Rahimi H, Moghaddas F, Rajebi H (2020) Predictive value of CT in the short-term mortality of Coronavirus Disease 2019 (COVID-19) pneumonia in nonelderly patients: a case-control study. Eur J Radiol 132:1–5
Zhou S, Chen C, Hu Y, Lv W, Ai T, Xia L (2020) Chest CT imaging features and severity scores as biomarkers for prognostic prediction in patients with COVID-19. Ann Transl Med 8:1–14
Wang C, Horby PW, Hayden FG, Gao GF (2020) A novel coronavirus outbreak of global health concern. Lancet 395:470–473
Bernheim A, Mei X, Huang M et al (2020) Chest CT findings in coronavirus disease-19 (COVID-19): relationship to duration of infection. Radiology. https://doi.org/10.1148/radiol.2020200463
Pan F, Ye T, Sun P et al (2020) Time course of lung changes on chest CT during recovery from 2019 novel coronavirus (COVID-19) pneumonia. Radiology. https://doi.org/10.1148/radiol.2020200370
Guan WJ, Ni ZY, Hu Y et al (2019) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Coronavirus disease (COVID-19)–Statistics and research. https://ourworldindata.org/ coronavirus. Accessed 8 October 2021.
Ai T, Yang Z, Hou H et al (2020) Correlation of chest CT and RT-PCR testing for coronavirus disease 2019 (COVID-19) in China: a report of 1014 cases. Radiology 296:32–40
Pang C, Hou Q, Yang Z, Ren L (2021) Chest computed tomography as a primary tool in COVID-19 detection: an update meta-analysis. Clin Transl Imaging. https://doi.org/10.1007/s40336-021-00434-z
Abdel-Tawab M, Basha MAA, Mohamed IAI, Ibrahim HM (2021) A simple chest CT score for assessing the severity of pulmonary involvement in COVID-19. Egypt J Radiol Nucl Med 52:1–10
Acknowledgements
The authors would like to thank Dr. Sinem Akkaya Işık, Dr. Okan Dilek, and Dr. Vecihe Bayrak for preparing the data we need in this study.
Funding
This research has been supported by Ilam University of Medical Sciences (Ethics code: IR.MEDILAM.REC.1400.188).
Author information
Authors and Affiliations
Contributions
SSZ provided study concepts and was involved in study design, the definition of intellectual content, literature search, data acquisition, data analysis, statistical analysis, manuscript preparation, manuscript editing, and manuscript review; NN provided study concepts and was involved in the definition of intellectual content, literature search, data acquisition, manuscript preparation, and manuscript review; DR took part in literature search, data acquisition, manuscript preparation, and manuscript review. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zakariaee, S.S., Naderi, N. & Rezaee, D. Prognostic accuracy of visual lung damage computed tomography score for mortality prediction in patients with COVID-19 pneumonia: a systematic review and meta-analysis. Egypt J Radiol Nucl Med 53, 64 (2022). https://doi.org/10.1186/s43055-022-00741-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-022-00741-z