Abstract
Purpose
Chest computed tomography (CT) is a high-sensitivity diagnostic tool for depicting interstitial pneumonia and may lay a critical role in the evaluation of the severity and extent of pulmonary involvement. In this study, we aimed to evaluate the association of chest CT severity score (CT-SS) with the mortality of COVID-19 patients using systematic review and meta-analysis.
Methods
Web of Science, PubMed, Embase, Scopus, and Google Scholar were used to search for primary articles. The meta-analysis was performed using the random-effects model, and odds ratios (ORs) with 95% confidence intervals (95%CIs) were calculated as the effect sizes.
Results
This meta-analysis retrieved a total number of 7106 COVID-19 patients. The pooled estimate for the association of CT-SS with mortality of COVID-19 patients was calculated as 1.244 (95% CI 1.157–1.337). The pooled estimate for the association of CT-SS with an optimal cutoff and mortality of COVID-19 patients was calculated as 7.124 (95% CI 5.307–9.563). There was no publication bias in the results of included studies. Radiologist experiences and study locations were not potential sources of between-study heterogeneity (both P > 0.2). The shapes of Begg’s funnel plots seemed symmetrical for studies evaluating the association of CT-SS with/without the optimal cutoffs and mortality of COVID-19 patients (Begg’s test P = 0.945 and 0.356, respectively).
Conclusions
The results of this study point to an association between CT-SS and mortality of COVID-19 patients. The odds of mortality for COVID-19 patients could be accurately predicted using an optimal CT-SS cutoff in visual scoring of lung involvement.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since the outbreak of the COVID-19 pandemic at the end of 2019, a total of 5,414,769 deaths had been reported all over the world [1]. Symptoms of this new infectious disease include fever, respiratory illness, lymphopenia, and pneumonia of unknown etiology [2,3,4,5,6,7]. The severity of pneumonia caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) could explain the high mortality of the infected subjects [8]. Given the mortality rate of COVID-19, it is very important to find predictors of poor prognosis to properly deal with COVID-19 patients. Early identification of patients at higher risk of death would help to recognize the patients with a higher need for intensive care. Improvement of the patient's management and their outcomes would be the main results of this clinical decision-making optimization [9].
However, the reverse-transcriptase polymerase chain reaction (RT-PCR) test is the main standard of reference for confirming COVID-19, CT is used as a fundamental complementary diagnostic approach [8]. Chest computed tomography (CT) is a high-sensitivity diagnostic tool for depicting interstitial pneumonia [10] and may lay a critical role in the evaluation of the severity and extent of pulmonary involvement. Bilateral multiple lobar ground-glass opacity and consolidation were the main chest CT findings on admission [11, 12]. Other chest CT manifestations include crazy-paving patterns, multi-lobar involvement, and increasing lung consolidations with disease progress [12].
CT severity score (CT-SS) is the suggested index to evaluate the severity of pulmonary involvement. In several studies, it was reported that there were significantly higher CT-SS magnitudes in deceased patients as compared to survivors. A significant correlation between CT-SS and mortality of the COVID-19 patients was also observed. Therefore, the extent of the lung lesions in early CT images after symptom onset could be considered as a potential predictor of patient mortality.
To our knowledge, a dedicated systematic review and meta-analysis study has not yet been performed for the assessment of the prognostic value of CT-SS to predict mortality in COVID-19 patients. In this study, we aimed to evaluate the association of CT-SS with mortality of COVID-19 patients using systematic review and meta-analysis.
Materials and methods
Protocol of the systematic review and meta-analysis
This systematic review was performed following a predefined protocol and reported in accordance with the preferred reporting items for systematic reviews and meta-analyses (PRISMA) checklist [13]. The study was approved by the local Medical Ethical Committee (approved number. IR.ABADANUMS.REC.1400.087).
Information sources and search strategies
Web of Science, PubMed, Embase, Scopus, and Google Scholar were used to search for primary articles evaluating the association of CT-SS with mortality of COVID-19 patients. These databases were searched between December 2019 and August 2021. We used the following MeSH and non-MeSH terms:
“COVID-19”, “SARS-CoV-2”, “2019 Novel Coronavirus”, “2019-nCoV”, “Wuhan virus”, “severe acute respiratory syndrome coronavirus 2”, “coronavirus disease 2019” in conjunction with “CT”, “computed tomography”, “Chest computed tomography”, “Chest CT”, “X-Ray CT Scan”, “X-Ray CAT Scan”, “CT scan”, “CAT scan”, “Mortality”, “Death”, “decease*”, “died”, and “dead. Boolean operators (NOT, AND, OR) were also used in succession. The references of the included studies were also screened for other possible additional publications. Case reports, editorials, commentaries, and opinions were not included in the meta-analysis.
Eligibility criteria
The inclusion criteria for the meta‐analysis were as follows: (a) studies on patients with laboratory‐confirmed COVID‐19 disease; (b) studies on patients with CT examination on admission/ after triage; (c) studies that have reported the association of CT-SS with mortality of COVID‐19 patients using OR; (d) studies in which CT-SS scores have been determined based on the visual assessment of the extent of lung involvements in initial CT images; and (e) original research with any type of study designs. Exclusion criteria were: (a) The full texts of studies were not available; (b) studies that have reported the association of CT-SS with mortality of COVID‐19 patients using hazard ratio (HR); and (c) studies in which necessary parameters for OR calculation did not exist.
Study selection and data collection process
Data extraction was carried out independently by two reviewers (NN and HS). In case of disagreement, the consensus was reached by discussing it with a third reviewer (SSZ). The first author of the selected articles, publication year, country, age average, sample size, the gender ratio of males, OR for the association of CT-SS with mortality of COVID-19 patients, the total number of deceased and survivors, and other related information were extracted from the studies that have been provided for the meta-analysis process. The characteristics of included studies are presented in Table 1.
Assessment of quality of studies
The Newcastle–Ottawa Scale (NOS) was used to assess the quality and risk of bias of the included studies. The NOS tool determines the quality of a case–control study according to selection (0–4 points), comparability (0–2 points), and exposure (0–3 points) definitions. The studies were categorized as good quality if they scored ≥ 7 points, fair quality if they scored 5–6 points, and poor quality if they scored < 5 points. The assessment of study quality was independently conducted by two investigators (HS and NN) and any discrepancy was resolved through discussion.
Summary measures and synthesis of results
Data analysis was performed using Stata version 11.0 (Stata Corporation, College Station, TX, USA). ORs were used for the analysis of a pooled effect size. In this study, the association between CT-SS (exposure) and mortality of COVID-19 patients (outcome) was evaluated. The OR represents the odds of mortality for COVID-19 patients with CT-SS > the considered threshold in each study, compared to the odds of mortality for COVID-19 patients with CT-SS < the threshold. Since we have a ratio of mortality probability in patients with CT-SS > the threshold to mortality probability in patients with CT-SS < the threshold, the range of scores in each study do not affect the results. Therefore, the difference between scoring systems has no role in calculating OR and has not been considered.
For included and pooled studies, data were presented as ORs with 95% CIs. Between-studies heterogeneity was evaluated using Cochran’s Q test and the Inconsistency index (I2) test.
I2 > 50% was considered as the apparent heterogeneity between the studies and the random-effects model (Der Simonian and Laird method) was adopted. For those analyses with I2 < 50%, the fixed-effect model (Mantel–Haenszel model) was used. For evaluation of publication bias among studies, a visual inspection of the generated funnel plot was employed.
Results
Study selection
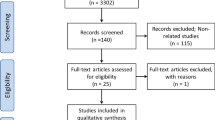
Figure 1 shows the flowchart of the study selections in the systematic review processes. A total of 7463 records were identified in the initial literature search. After removing duplicate studies, 3302 studies were screened based on title and abstract. Of the 3302 records, 3162 publications were excluded after the first screening. Because of data presentation in HR and the insufficient data for calculation of OR and 95% CI two papers were excluded. Based on inclusion criteria, 28 studies (which are detailed in Table 1) were included in the meta-analytical processes that reported the association of CT-SS with mortality of COVID-19 patients.
Characteristics and quality of the included studies
The characteristics of the included studies are presented in Table 1. The first author, publication year, country, age average, sample size, the gender ratio of males, and the method of image interpretation of the included articles are presented in the table. This meta-analysis retrieved a total number of 7106 COVID-19 patients.
The quality of studies were assessed using the NOS tool for case–control studies and the results are shown in Table 1. The scores were as follows: 9/9 score [2 studies (7%)]; 8/9 score [2 studies (7%)]; and 7/9 score [24 study (86%)]. The cases and controls could be compared for only four studies based on the design or analysis controlled for age or other confounding variables. All studies were categorized as good quality (scores of ≥ 7). Therefore, quality of the included studies were not the source of heterogeneity among the studies.
Risk of bias within studies
The p value obtained from the χ2 test of heterogeneity could be used to determine the presence of heterogeneity between studies. The heterogeneity is significant when there is a low p value. The p value obtained from the χ2 test of heterogeneity were 0.008 and < 0.0001 for studies evaluating the association of CT-SS with mortality of COVID-19 patients and studies predicting mortality of COVID-19 patients using the optimal CT-SS cutoffs, respectively. Moreover, the I2 test for studies evaluating the association of CT-SS with mortality of COVID-19 patients and studies predicting mortality of COVID-19 patients using the optimal CT-SS cutoffs were calculated as 65.5 and 63.4%, respectively. Therefore, the random-effects model of the meta-analysis was applied for evaluating the associations of both CT-SSs with mortality of COVID-19 patients.
Synthesis of results
The forest plot for the included studies reporting the association of CT-SS with mortality of COVID-19 patients is presented in Fig. 2. In addition to the effect sizes (in OR) and 95% CIs of the studies, the overall effect size and its 95% CI have also been presented in this plot. The pooled estimate for the association of CT-SS with mortality of COVID-19 patients was calculated as 1.244 (95% CI 1.157–1.337). The pooled estimate is significant.
The forest plot for the included studies predicting mortality of COVID-19 patients using the optimal CT-SS cutoffs is presented in Fig. 3. In addition to the effect sizes (in OR) and 95% CI magnitudes of the studies, the overall effect size and its 95% CI have also been presented in this plot. The pooled estimate for the association of CT-SS with an optimal cutoff and mortality of COVID-19 patients was calculated as 7.124 (95% CI 5.307–9.563). The pooled estimate is significant.
Risk of bias across studies
In Fig. 4, the funnel plot seemed symmetrical in shape (P = 0.941) demonstrating the absence of publication bias in the results of included studies evaluating the association of CT-SS and mortality of COVID-19 patients.
Funnel plot for 7 included studies. For interpretation of any publication bias among studies, a visual inspection of the generated funnel plot was employed. The funnel plot seemed symmetrical in shape (P = 0.941) demonstrating the absence of publication bias in the results of included studies evaluating the association of CT-SS and mortality of COVID-19 patients. In this plot, the X and Y axes represent ORs and standard errors, respectively
In Fig. 5, the shape of the Begg’s funnel plot seemed symmetrical (P = 0.365) which indicates the absence of publication bias in the results of included studies predicting mortality of COVID-19 patients using the optimal CT-SS cutoffs.
Funnel plot for 25 included studies. For interpretation of any publication bias among studies, a visual inspection of the generated funnel plot was employed. The funnel plot seemed symmetrical in shape (P = 0.941) demonstrating the absence of publication bias in the results of included studies evaluating the association of CT-SS with an optimal cutoff and mortality of COVID-19 patients. In this plot, the X and Y axes represent ORs and standard errors, respectively
To explore the possible source of between-study heterogeneity, meta-regression analysis was conducted on radiologist experiences. Results showed that radiologist experiences did not affect the prognostic value of CT-SS to predict mortality in COVID-19 patients (P = 0.404). In subgroup analysis, study locations were also investigated to evaluate another possible source of heterogeneity among the studies. The test for subgroup differences indicated that there is no statistically significant subgroup effect (P = 0.229, analysis not presented), suggesting that study location does not modify the prognostic value of CT-SS to predict mortality in COVID-19 patients.
Discussion
In the last 2 years, countless infections and deaths were reported due to the outbreak of the COVID-19 pandemic. Effective management and treatment of the infected subjects might be possible by understanding the mechanism of severe/critical conditions and identification of their imaging and clinical features [37,38,39]. Radiological and clinical features of COVID-19 pneumonia have been investigated in several case series [40, 41]. Radiological manifestations of COVID-19 lesions resemble those of SARS and MERS pneumonia, which may be related to their similar pathogenesis [42].
The pneumonia lesions mostly involved the bilateral lungs and were located in the peripheral area of the lungs. The radiological lung injury abnormalities could be manifested on all five lobes. In the early period of virus infection, GGO without consolidation is the most common radiological sign. Consolidation is more commonly seen in severely ill subjects, which indicates that there has been a correlation between the consolidative lesions and disease severity [43, 44].
In addition to these radiological manifestations, lung lesion extension has also a relatively strong association with disease severity and patient outcome. Deceased patients had higher CT severity scores compared to survivors. Therefore, CT-SS as a prognostic factor could help to identify the high-risk patients and give physicians a better insight to manage patients with COVID-19.
In this study, we investigated the association of CT-SS with the mortality of COVID-19 patients by conducting a meta-analysis. Although there are original articles describing the association of CT-SS with mortality of COVID-19 patients and predicting mortality of COVID-19 patients using the optimal CT-SS cutoffs, no meta-analysis study has to date been carried out on this aspect. To the best of our knowledge, this report is the first meta-analysis describing the association of CT-SS with mortality of COVID-19 patients to provide appropriate clinical evidence for the prognosis of COVID-19.
In the current study, the association of CT-SS with mortality of COVID-19 patients was investigated using meta-analysis and the final pooled OR was calculated as 1.244 (95% CI 1.157–1.337). The result confirms that there is a direct relationship between CT-SS and mortality of the COVID-19 patients. For the COVID-19 patients with higher CT severity scores, there is a higher odds of mortality. The results also showed that there is no heterogeneity among the included studies. In further meta-regression and subgroup analyses, radiologist experiences and study locations were, respectively, investigated to evaluate the possible sources of heterogeneity among the studies. The results indicated that study location and radiologist experience don’t modify the prognostic value of CT-SS to predict mortality in COVID-19 patients.
The time interval between the initial CT and the symptom onset might be another possible source of between-study heterogeneity. As indicated by a comprehensive meta-analysis of 9907 confirmed patients, initial normal chest CT scans after symptom onset can rapidly change and lung damages would be more pronounced in the following days [45]. The possible interaction between these time intervals and the prognostic performance of the CT-SS index cannot be investigated, because individual participant data were not available. In this study, the possible selection bias as the study limitation should be acknowledged. However, we tried to avoid any selection bias and there was no language limitation in the search strategy. There is potential for selection bias, because only published studies written in the English language were retrieved.
In this study, the prognostic efficiency of optimal CT-SS cutoffs to predict mortality of COVID-19 patients was also investigated using meta-analysis and the final pooled OR was calculated as 7.124 (95% CI 5.307–9.563). The result confirms that an optimal cutoff for CT-SS can accurately predict the prognosis of the COVID-19 patients. From the results, it could be concluded that the simple CT-SS has important clinical value and is of great significance for the diagnosis of patients who need more aggressive treatment. Thus, in addition to the typical radiological manifestations, CT-SS must be routinely included in the radiological reports for COVID-19 patients. This score could provide vital information about patient prognosis and might be potentially used to guide clinical management.
Study limitations
CT-SS as a valuable factor to predict mortality in COVID-19 patients has some limitations that must be acknowledged. A wide scoring range from 20 to 40 regions has been used for the quantification of COVID-19 pneumonia. This diversity in scoring approaches makes assessments more difficult. Therefore, CT-SS would be inherently complex and time-consuming. The similar segmented sectors of the right and left lungs have different sizes, because the right lung is larger than the left lung. For quantitative and semi-quantitative studies, these differences must be taken into account and dedicated software is required [46].
For included studies, chest CT scans were performed on all suspected individuals after triage. The interval between the onset of the symptom and the time of imaging is different for each patient. These data were not available to the authors. Therefore, the issue of time of imaging and their related implications on the scoring scheme could not be addressed.
Various scoring methods to quantify pulmonary involvement have been proposed in studies (including lung involvement by GGO or consolidations). Given that in the studies included in the meta-analysis, there are not enough results related to these scores; we cannot separately combine them and discuss their extent of lung involvement.
Conclusions
The results of this study point to an association between CT-SS and mortality of COVID-19 patients. The odds of mortality for COVID-19 patients could be accurately predicted using an optimal CT-SS cutoff in visual scoring of lung involvement.
Availability of data and materials
All data generated or analyzed during this study are included in the article.
Abbreviations
- COVID-19:
-
Coronavirus disease 2019
- SARS-CoV-2:
-
Severe acute respiratory syndrome coronavirus 2
- CT-SS:
-
Chest CT severity score
- CT:
-
Computed tomography
- CI:
-
Confidence interval
- RT-PCR:
-
Reverse-transcriptase polymerase chain reaction
- OR:
-
Odds ratio
- HR:
-
Hazard ratio
References
Worldometer COVID-19 Data (2021). https://www.worldometers.info/coronavirus/?zarsrc=130. Accessed 8 Oct 2021
Yuan M, Yin W, Tao Z et al (2020) Association of radiologic findings with mortality of patients infected with 2019 novel coronavirus in Wuhan, China. PLoS ONE 15:1–10
Guan W-j, Ni Z-y, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382:1708–1720
Cheng Z, Lu Y, Cao Q et al (2020) Clinical features and chest CT manifestations of coronavirus disease 2019 (COVID-19) in a single-center study in Shanghai, China. AJR Am J Roentgenol 215:121–126
Tahvildari A, Arbabi M, Farsi Y et al (2020) Clinical features, diagnosis, and treatment of COVID-19 in hospitalized patients: a systematic review of case reports and case series. Front Med 7:1–10
Xiong Y, Sun D, Liu Y et al (2020) Clinical and high-resolution CT features of the COVID-19 infection: comparison of the initial and follow-up changes. Invest Radiol 55:1–8
Chen H, Ai L, Lu H et al (2020) Clinical and imaging features of COVID-19. Radiol Infect Dis 7:43–50
Guillo E, Gomez IB, Dangeard S et al (2020) COVID-19 pneumonia: diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur J Radiol 131:1–5
Besutti G, Ottone M, Fasano T et al (2021) The value of computed tomography in assessing the risk of death in COVID-19 patients presenting to the emergency room. Eur Radiol 31:9164–9175
Hansell DM, Bankier AA, MacMahon H et al (2008) Fleischner society: glossary of terms for thoracic imaging. Radiology 246:697–722
Raoufi M, Naini SAAS, Azizan Z et al (2020) Correlation between chest computed tomography scan findings and mortality of COVID-19 cases: a cross sectional study. Arch Acad Emerg Med 8:1–7
Kazemi MA, Ghanaati H, Moradi B et al (2020) Prognostic factors of initial chest CT findings for ICU admission and mortality in patients with COVID-19 pneumonia. Iran J Radiol 17:1–10
Liberati A, Altman DG, Tetzlaff J et al (2009) The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol 62:1–34
Abdollahi I, Nabahati M, Javanian M et al (2021) Can initial chest CT scan predict status and clinical outcomes of COVID-19 infection? A retrospective cohort study. Egypt J Radiol Nucl Med 52:1–10
Angeli E, Dalto S, Marchese S et al (2021) Prognostic value of CT integrated with clinical and laboratory data during the first peak of the COVID-19 pandemic in Northern Italy: a nomogram to predict unfavorable outcome. Eur J Radiol 137:1–10
Bayrak V, Durukan NŞ, Aydemir FD et al (2021) Risk factors associated with mortality in ıntensive care COVID-19 patients: the importance of chest CT score and intubation timing as risk factors. Turk J Med Sci 51:1665–1674
Cao Y, Han X, Gu J et al (2020) Prognostic value of baseline clinical and HRCT findings in 101 patients with severe COVID-19 in Wuhan, China. Sci Rep 10:1–13
Charpentier E, Soulat G, Fayol A et al (2021) Visual lung damage CT score at hospital admission of COVID-19 patients and 30-day mortality. Eur Radiol 31:8354–8363
Chon Y, Kim JY, Suh YJ et al (2020) Adverse initial CT findings associated with poor prognosis of coronavirus disease. J Korean Med Sci 35:1–12
Dilek O, Demirel E, Akkaya H et al (2021) Different chest CT scoring systems in patients with COVID-19: could baseline CT be a helpful tool in predicting survival in patients with matched ages and co-morbid conditions? Acta Radiol 63:615–622
Hajiahmadi S, Shayganfar A, Janghorbani M et al (2021) Chest computed tomography severity score to predict adverse outcomes of patients with COVID-19. Infect Chemother 53:308–318
Isik SA, Samanci C, Yesildal M et al (2021) Relationship of radiological stage with laboratory parameters and mortality in COVID-19 pneumonia. Acta Med Mediterr 37:445–451
Khosravi B, Aghaghazvini L, Sorouri M et al (2021) Predictive value of initial CT scan for various adverse outcomes in patients with COVID-19 pneumonia. Heart Lung 50:13–20
Kimura-Sandoval Y, Arévalo-Molina ME, Cristancho-Rojas CN et al (2021) Validation of chest computed tomography artificial intelligence to determine the requirement for mechanical ventilation and risk of mortality in hospitalized coronavirus disease-19 patients in a tertiary care center in Mexico City. Rev Invest Clin 73:111–119
Li H, Luo S, Zhang Y et al (2021) Longitudinal chest CT Features in severe/critical COVID-19 cases and the predictive value of the initial CT for mortality. J Inflamm Res 14:1111–1124
Li K, Chen D, Chen S et al (2020) Predictors of fatality including radiographic findings in adults with COVID-19. Respir Res 21:1–10
Li K, Wu J, Wu F et al (2020) The clinical and chest CT features associated with severe and critical COVID-19 pneumonia. Invest Radiol 55:1–5
Li Y, Yang Z, Ai T et al (2020) Association of “initial CT” findings with mortality in older patients with coronavirus disease 2019 (COVID-19). Eur Radiol 30:6186–6193
Lieveld AW, Azijli K, Teunissen BP et al (2021) Chest CT in COVID-19 at the ED: validation of the COVID-19 reporting and data system (CO-RADS) and CT severity score: a prospective, multicenter, observational study. Chest 159:1126–1135
Mirza-Aghazadeh-Attari M, Zarrintan A, Nezami N et al (2020) Predictors of coronavirus disease 19 (COVID-19) pneumonitis outcome based on computed tomography (CT) imaging obtained prior to hospitalization: a retrospective study. Emerg Radiol 27:653–661
Mohamed IA, Hasan HA, Abdel-Tawab M (2021) CT characteristics and laboratory findings of COVID-19 pneumonia in relation to patient outcome. Egypt J Radiol Nucl Med 52:1–7
Ruch Y, Kaeuffer C, Ohana M et al (2020) CT lung lesions as predictors of early death or ICU admission in COVID-19 patients. Clin Microbiol Infect 26:5–8
Salahshour F, Mehrabinejad M-M, Toosi MN et al (2021) Clinical and chest CT features as a predictive tool for COVID-19 clinical progress: introducing a novel semi-quantitative scoring system. Eur Radiol 31:5178–5188
Salvatore C, Roberta F, Angela DL et al (2021) Clinical and laboratory data, radiological structured report findings and quantitative evaluation of lung involvement on baseline chest CT in COVID-19 patients to predict prognosis. Radiol Med 126:29–39
Tabatabaei SMH, Rahimi H, Moghaddas F et al (2020) Predictive value of CT in the short-term mortality of coronavirus disease 2019 (COVID-19) pneumonia in nonelderly patients: a case-control study. Eur J Radiol 132:1–5
Zhou S, Chen C, Hu Y et al (2020) Chest CT imaging features and severity scores as biomarkers for prognostic prediction in patients with COVID-19. Ann Transl Med 8:1–14
Therapeutic Management of Hospitalized Adults with COVID-19 (2021). https://www.covid19treatmentguidelines.nih.gov/management/clinical-management/hospitalized-adults--therapeutic-management/. Accessed 8 Oct 2021
Lo Bianco G, Di Pietro S, Mazzuca E et al (2020) Multidisciplinary approach to the diagnosis and in-hospital management of COVID-19 infection: a narrative review. Front Pharmacol 11:1–17
Shayganfar A, Sami R, Sadeghi S et al (2021) Risk factors associated with intensive care unit (ICU) admission and in-hospital death among adults hospitalized with COVID-19: a two-center retrospective observational study in tertiary care hospitals. Emerg Radiol 28(4):691–697
Tabatabaei SM, Rajebi H, Moghaddas F et al (2020) Chest CT in COVID-19 pneumonia: what are the findings in mid-term follow-up? Emerg Radiol 27(6):711–719
Vernuccio F, Giambelluca D, Cannella R et al (2020) Radiographic and chest CT imaging presentation and follow-up of COVID-19 pneumonia: a multicenter experience from an endemic area. Emerg Radiol 27(6):623–632
Wan S, Li M, Ye Z et al (2020) CT manifestations and clinical characteristics of 1115 patients with coronavirus disease 2019 (COVID-19): a systematic review and meta-analysis. Acad Radiol 27:910–921
Pang C, Hou Q, Yang Z et al (2021) Chest computed tomography as a primary tool in COVID-19 detection: an update meta-analysis. Clin Transl Imaging. 9:341–351
Vafea MT, Atalla E, Kalligeros M et al (2020) Chest CT findings in asymptomatic cases with COVID-19: a systematic review and meta-analysis. Clin Radiol 75:33–39
Zarifian A, Nour MG, Rezayat AA et al (2021) Chest CT findings of coronavirus disease 2019 (COVID-19): a comprehensive meta-analysis of 9907 confirmed patients. Clin Radiol 70:101–110
Abdel-Tawab M, Basha MAA, Mohamed IAI, Ibrahim HM (2021) A simple chest CT score for assessing the severity of pulmonary involvement in COVID-19. Egypt J Radiol Nucl Med 52:1–10
Acknowledgements
Authors would like to thank the research affair of Medicine faculty of Abadan University of Medical Sciences. We would also like to thank Dr. Sinem Akkaya Işık, Dr. Okan Dilek, and Dr. Vecihe Bayrak for preparing the data needed in this study.
Funding
This study was funded by Abadan University of Medical Sciences (grant number 1304, 10 Nov 2021).
Author information
Authors and Affiliations
Contributions
SSZ: content planning, literature search and review, data collection, manuscript writing and editing, and meta-analysis. HS: content planning, literature search and review, data collection, and manuscript editing. NN: literature search and review, data collection, and manuscript editing. HK-A: project development, literature review, manuscript writing and editing, and meta-analysis. MS: literature review and manuscript editing.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval and consent to participate
The study was approved by the local Medical Ethical Committee (approved number. IR.ABADANUMS.REC.1400.087).
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Zakariaee, S.S., Salmanipour, H., Naderi, N. et al. Association of chest CT severity score with mortality of COVID-19 patients: a systematic review and meta-analysis. Clin Transl Imaging 10, 663–676 (2022). https://doi.org/10.1007/s40336-022-00512-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40336-022-00512-w