Abstract
Background
Pilocytic astrocytoma, medulloblastoma, and ependymoma are the most common pediatric CNS tumors seen at posterior cranial fossa and final diagnosis obtained by histopathology after surgical excision. Routine MRI study gives an idea about site and extension of the tumors but provide a little information about type and grade of tumors. ADC ratio had high sensitivity and specificity in differentiation between these tumors as regard type and grade according to tumor cellularity.
Patients and methods
Prospective study conducted on thirty pediatric patients (11 males and 19 females) with CNS posterior fossa masses, their ages ranged from 2 to 17 years (mean age of 8.7 years), conventional MRI, DWI, ADC value, and ADC ratio were done for all patients.
Results
ADC values were significantly different between pilocytic astrocytomas (1.43 ± 0.28 × 10−3) and medulloblastomas (0.71 ± 0. 21 × 10−3) with a P value < 0.001, also there was a significant difference when comparing medulloblastomas (0.71 ± 0.21 × 10−3) with ependymomas (1.04 × 10−3 ± 0.21) with a P value < 0.001. ADC ratio at a cutoff > 1.7 showed significant good power of discrimination of astrocytoma (AUC = 0.85) from ependymoma with 87.5% sensitivity and 93.3% specificity. Similarly, at cutoff ≤ 1.6-> 1.2 was a significant good predictor of ependymoma (AUC = 0.85) with 87.8% sensitivity and 99.5% specificity. While, ADC ratio ≤ 1.2 was significant excellent discriminator of medulloblastoma (AUC = 0.99) with 100% sensitivity and 90% specificity.
Conclusion
ADC ratio is a simple way used in distinguishing juvenile pilocytic astrocytoma, ependymoma, and medulloblastoma, which are the most frequent pediatric posterior fossa tumors. Cutoff ADC ratio of more than 1.7 characteristic of JPA with 87.5% sensitivity and 93.3% specificity, ADC ratio less than 1.1 characteristic of medulloblastoma with 100% sensitivity and 90% specificity. ADC ratios more than 1.1 and less than 1.7 characteristic of ependymoma with 87.8% sensitivity and 99.5% specificity. We recommended ADC ratio as a routine study in evaluation of pediatric CNS posterior fossa tumors.
Similar content being viewed by others
Introduction
Pediatric central nervous system tumors accounting for approximately 20 to 25% of all primary pediatric tumors and representing the second most common cancer after hematological malignancies [1]. Cranial posterior fossa tumors accounting for 54-70% of all brain tumors in pediatrics [2].
Ependymomas, pilocytic astrocytomas (PAs), and medulloblastomas (MBs) are the most important key players in the first 2 years of life [3]. Definitive diagnosis for pediatric brain tumors proved mainly after surgical excision by histopathology and uncommonly by preoperative biopsy [4].
MRI is the imaging modality of choice for the evaluation of pediatric intracranial tumors. Conventional T1, T2, and contrast-enhanced T1-weighted imaging (WI) provide information about site, morphology, extension, and mass effect of the tumor as well as the presence of hemorrhage or ectatic vessels within the tumor and a little information about its nature as benign or malignant [5].
Magnetic resonance imaging provides limited information as regard type and grading of the tumors. Diffusion-weighted (DW) MR imaging provided additional information derived from a microscopic motion of the water proton, which not obtained by using conventional MRI. DW imaging has been applied for the assignment of tumor grades or differentiation of tumor types, as well as for the diagnosis of other brain SOLs [6].
Visual inspection of diffusion-weighted images has been reported as less accurate in differentiating types and grades of tumors, whereas an important trend has appeared toward the use of quantitative diffusion imaging technique [7].
Measurements of the ADC values of the brain tumors were very useful in tumor assessment. Variation in water content and diffusivity which was found in tumors for various reasons (e.g., vasogenic edema) likely provide information that is not readily available from conventional MR imaging [8]. ADC values could accurately distinguish pediatric cranial posterior fossa tumors especially pilocytic astrocytoma and medulloblastomas due to significant differences in tumor cellularity. Higher grade tumors have more restricted diffusion due to their higher cellularity, resulting in a good correlation between ADC values and tumor grade [9].
Apparent diffusion coefficient (ADC) values are significantly lower in MBs due to its high cell density (P < 0.001). JPA consists of low cell density with wider extracellular space. Thus, they exert less restricted diffusion. Most ependymomas in the posterior fossa are classic (grade II) ependymomas present with no or mild restricted diffusion [10].
ADC values were significantly higher in pilocytic astrocytomas than in ependymomas and medulloblastomas [11]. ADC of ependymomas was higher than that of PNETs, and there was no overlapping that makes preoperative differentiation between ependymomas and medulloblastomas possible [8].
Some cleavage between the brain stem and the tumor can be perceived on MRI T2 sequences; however, the cleavage may be imperceptible. The cleft appears more distinct on ADC map sequences. The diffusion restriction seen on the ADC map sequence along the interface between a posterior fossa medulloblastoma and the brain stem and/or cerebellar peduncles can predict infiltration of these structures [12].
Apparent diffusion coefficient ratio of pediatric cranial posterior fossa tumors to normal-appearing cerebellar parenchyma allowed preoperative diagnosis of JPA, medulloblastoma, and ependymoma with high sensitivity and specificity [13].
Mean ADC values for juvenile pilocytic astrocytoma, ependymoma, and medulloblastoma were 1.42 ± 0.27 × 10−3, 1.03 ± 0.21 × 10−3, and 0.707 ± 0.21 × 10−3 mm2/s respectively (P ≤ 0.001). ADC ratios were found to be 1.95 ± 0.30, 1.50 ± 0.20, and 1.02 ± 0.30 for juvenile pilocytic astrocytoma, ependymoma, and medulloblastoma respectively [14].
The present study aimed to evaluate diagnostic accuracy of ADC ratio in differentiation between pilocytic astrocytoma, medulloblastoma, and ependymoma which were the most common pediatric CNS tumors as cMRI provide limited information about types and grades of the tumors.
Patients and methods
Patients population
This prospective study was carried out on 30 pediatric patients (11 male and 19 female), their ages ranged from 2 to 17 years (mean age of 8.7 years), throughout period extending from May 2018 to August 2019 who underwent routine cMRI study and proved to have one of the three common pediatric posterior fossa tumors based on our inclusion criteria. Approval of the Research Ethics Committee (REC) and written consent were obtained from all participants in the study. Privacy of all patient data was guaranteed.
Inclusion criteria include pediatric patients with brain posterior fossa mass, both sexes were included. Exclusion criteria include patients with post-operative recurrent or residual tumors or with contraindication to MRI as (non-compatible metallic implants, aneurysmal clips, cardiac pacemaker, cochlear implants).
All patients were subjected to the following:
Data collection and clinical examination
MR imaging
Routine MRI sequences, diffusion-weighted MRI, ADC value, and ADC ratio between tumor area and normal cerebellar tissue were done for all patients. Magnetic resonance imaging was performed on a 1.5 Tesla MRI system (GE sigma explorer). Total study time ranged from 15 to 20 min. Fasting 4 h before the scan was required for patients needed sedation\anesthesia (16 patients).
Routine MRI sequences
All cases were examined in supine position using the following routine sequences: Axial T1-WI (TR/TE = 520/15 ms), axial T2-weighted images (TR/TE = 3900/100 ms), axial fluid-attenuated inversion recovery images (TR/TE = 8000/90 ms, inversion time = 2000 ms), sagittal T1WI (TR/TE = 430/10 ms), post-contrast MRI was also performed after injecting (Gd-DTPA) intravenously at a dose of 0.2 ml/kg (0.1 mmol/kg body weight). Post-contrast T1WI in axial, sagittal, and coronal planes were obtained.
Diffusion-weighted imaging with apparent diffusion coefficient ratio calculation
-
An axial echo-planar spin-echo sequence with an average 5 mm slice thickness, b values of 0 and 1000 s/mm2 were done to obtain DW images.
-
Mean ADC values are the mean for multiple measures of ADC values. Region of interest (ROI) measurements were acquired in 3 consecutive slices from the solid component of the lesions avoiding hemorrhagic and necrotic areas to obtain average ADC value.
-
Average ADC value was divided by the measurement ADC value obtained from normal cerebellar white matter to obtain ADC ratio.
-
Standard mean ADC values were calculated automatically and expressed in 10−3 mm2/s.
Image interpretation
-
Interpretation of MR images and ADC maps of the patients were evaluated by two experienced radiologists without any histopathological data.
Histopathological examination
The final diagnosis either juvenile pilocytic astrocytoma (JPA), ependymoma, or medulloblastoma was obtained by histopathological examination after surgical excision.
Statistical analysis
The data were collected, tabulated, and statistically analyzed. Data were analyzed using the IBM SPSS software package version 20.0. Descriptive statistic was performed in a form of percentage and number for qualitative data. Specificity, sensitivity, and diagnostic accuracy of ADC ratio were calculated for diagnostic evaluation in distinguishing between pilocytic astrocytoma, ependymoma, and medulloblastoma.
The used tests were as follows:
Chi-square test: for categorical variables, to compare between different groups
Monte Carlo correction: correction for chi-square when more than 20% of the cells have expected count less than 5
Kruskal-Wallis test: for abnormally distributed quantitative variables, to compare between more than two studied groups, and post hoc (Dunn’s multiple comparisons test) for pairwise comparisons.
Results
Thirty pediatric patients (11 males and 19 females) with CNS posterior fossa masses proved to be juvenile pilocytic astrocytoma, ependymoma, and medulloblastoma by cMRI study. Their ages ranged from 2 to 17 years with a mean of 8.7 years, most of them were in age group 5-≤ 10 years representing 46.66% as listed in Table 1.
Histopathological examination of all lesions was done after excisional biopsy; 12 (40%) lesions were ependymoma (WHO grade IV), 8 (26.7%) lesions were pilocytic astrocytoma (WHO grade I), and 10 (33.3%) were medulloblastoma (WHO grade III). According to their histopathological examination, the astrocytoma group included 3 male and 5 female their ages ranged from 5 to 11 years with a mean age of 8 ± 2.3. The ependymoma group included 6 male and 6 female their ages ranged from 6 to 16 years with a mean age of 9.5 ± 4.5. The medulloblastoma group included 2 male and 8 female their ages ranged from 3 to 13 years with a mean age of 8.4 ± 3.7; the above findings were listed in Fig. 1 and Table 2.
Many pediatric patients suffering from more than one complains, 66.6% of them presented with headache, 70% presented with vomiting, 76.6% presented with visual symptoms, 10% presented with increase head circumference, 23.3% presented with brain stem symptoms, and 83.3% with cerebellar symptoms.
MRI findings in all patients revealed 30 posterior fossa SOL were detected in 30 patients. Four lesions at left cerebellar hemisphere (pilocytic astrocytoma by histopathology), 4 lesions at right cerebellar hemisphere (2 pilocytic astrocytoma and 2 medulloblastoma by histopathology), 2 lesions at right cerebellopontine angle (pilocytic astrocytoma by histopathology), and 20 lesions seen intraventricular within fourth ventricle (12 ependymoma and 8 medulloblastoma by histopathology). Eighteen lesions were solid by histopathology (8 medulloblastoma and 10 ependymoma). Twelve lesions were mixed cystic and solid by histopathology (8 pilocystic astrocytoma, 2 medulloblastoma, and 2 ependymoma).
MRI diffusion-weighted image was done for all the studied cases; 13 lesions showed free diffusion (8 pilocytic astrocytoma and 5 ependymoma by histopathology) and 17 lesions showed restricted diffusion (7 ependymoma and 10 medulloblastoma by histopathology). Restricted diffusion was significantly more frequent in ependymoma and medulloblastoma, while free diffusion was more significantly frequent in pilocytic astrocytoma.
All studied patients were divided into 3 groups according to mean ADC value, (group 1) with mean ADC value > (1.43 ± 0.28 × 10−3) that predict pilocytic astrocytoma, (group 2) with mean ADC value > (0.71 ± 0. 21 × 10−3)-≤ (1.40 ± 0.12 × 10−3) that predict ependymoma, (group 3) mean ADC value ≤ (0.71 ± 0. 21 × 10−3) that predict medulloblastoma.
Group (1) mean ADC value > (1.43 ± 0.28 × 10−3) was detected in 6 lesions proved to be pilocytic astrocytoma by histopathology. Group (2) mean ADC value from > (0.71 ± 0. 21 × 10−3) - ≤ (1.43 ± 0.28 × 10−3) were detected in 14 lesions; 12 of them were ependymoma and 2 lesions were pilocytic astrocytoma by histopathology. Group (3) mean ADC value ≤ (0.71 ± 0.21 × 10−3) were detected in 10 lesions proved to be medulloblastoma by histopathologically. These results were statistically significant with a P value < 0.001 as showed in Fig. 2 and Table 3.
Mean ADC values were significantly different between pilocytic astrocytomas (1.43 ± 0.28 × 10−3) (mean ± SD) and medulloblastomas (0.71 ± 0. 21 × 10−3) with a P value < 0.001. We found a significant difference when comparing medulloblastomas (0.71 ± 0. 21 × 10−3) with ependymomas (1.04 × 10−3 ± 0.21) with a P value < 0.001 (Figs. 3, 4, 5, and 6).
A 6-year-old female patient complaining of headache. Axial T1 (a), axial T2 (b), axial FLAIR (c), coronal T1 post-contrast (d), sagittal T1 post-contrast (e)—a mixed cystic and solid SOL seen at posterior fossa involving cerebellar vermis and left cerebellar hemisphere measured 55 × 58 × 53 mm at its AP, width, and CC dimensions exerting mass effect inform of compressing 4th ventricle, the lesion mainly cystic displayed CSF signal intensity, solid nodule displayed low signal intensity at T1WI (a), high signal intensity at T2 (b), and FLAIR sequence (c) with mild contrast uptake (d), (e) seen only in the solid mural component. Axial DWI (f), axial ADC map (g)—the lesion showed free diffusion, ADC value of the lesion (1.490, 1.500, 1.570) × 10−3 mm2/s, average (1.500 × 10−3), ADC value of the normal cerebellum—(0.742, 0.743, 0.749) × 10−3 mm2/s, average 0.743 × 10−3 mm2/s, ADC ratio—2.3 that are consistent with cerebellar pilocytic astrocytoma confirmed by histopathology
A 4-year-old male patient complaining of vomiting. Axial T1 (a), axial T2 (b), axial FLAIR (c), coronal T1 (d), sagittal T1 (e)—posterior fossa midline SOL measured 45 × 40 × 40 mm at its AP, width, and CC diameters originating from the superior medullary valum and encroaching upon the 4th ventricular cavity with subsequent supratentorial hydrocephalic changes, hypointense to CSF on FLAIR, and exhibits inhomogeneous enhancement. Axial DWI (f), Axial ADC (g)—the lesion showed restricted diffusion, ADC ratio—0.9, ADC values of the lesion (0.693, 0.702, 0.709) × mm2/s, average 0.702 × 103 mm2/s, ADC value of the normal cerebellum—(0.742, 0.743, 0.749) × 103 mm2/s, average 0.743 × 103 mm2/s that are consistent with medulloblastoma confirmed by histopathology
A 7-year-old female patient complaining of headache and vomiting. Axial T1 (a), axial T2 (b), axial FLAIR (c), coronal T1 post contrast (d), sagittal T1 post-contrast (e)—posterior fossa SOL seen within fourth ventricle, extending to the right cerebellar hemisphere and right cerebellopontine angle. The mass measured 47 × 35 × 36 mm at its maximum AP, width, and CC diameters, it showed heterogeneous predominantly low SI at T1WI (a), high SI at T2 (b), and FLAIR sequence (c) with central breakdown, avid, and heterogenous enhancement after IV contrast injection (d), (e). Axial DWI (f), axial ADC (g)—the lesion showed restricted diffusion, ADC ratio—1.3, ADC values of the lesion—(1.006, 1.081, 1.087) × 103 mm2/s × 103 mm2/s, average 1.081 × 103, ADC value of the normal cerebellum—(0.789, 0.793, 0.797) × 103, average 0.793 × 103 mm2/s consistent with ependymoma confirmed by histopathology
An 8-year-old female patient complaining of vomiting. Axial T1 (a), axial T2 (b), axial FLAIR (c), coronal T1 post-contrast (d), sagittal T1 post-contrast (e)—posterior fossa intraventricular SOL seen filling the fourth ventricle and extending to the right cerebellum and left occipital lobe, measured 36 × 60 × 34 mm at its AP, width, and CC diameters. It showed heterogeneous predominantly low T1 SI (a), high SI at T2 WI (b), and FLAIR sequence (c) with cystic component, avid, and heterogenous enhancement after IV contrast injection (d), (e). Axial DWI (f), Axial ADC (g)—the lesion showed restricted diffusion, ADC ratio—0.7, ADC values of the lesion (0.597, 0.594, 0.543) × 103 mm2/s, average 0.597 × 103 mm2/s, ADC value of the normal cerebellum—(0.860, 0.863, 0.869) × 103, average 0.863 × 103 mm2/s consistent with medulloblastoma confirmed by histopathology
ADC ratio in the studied lesions was ranged from 0.7 to 2.20. the studied patients divided into three groups. Group (1) ADC ratio > (1.95 ± 0.30) that predict pilocytic astrocytoma. Group (2) ADC ratio > (0.93 ± 0.17)-≤ (1.95 ± 0.30) that predict ependymoma. Group (3) ADC ratio ≤ (0.93 ± 0.17) that predict medulloblastoma.
Group (1) ADC ratio > (1.95 ± 0.30) was detected in 7 lesions, all of them proved to be pilocytic astrocytoma by histopathology. Group (2) ADC ratio > (0.93 ± 0.17)-≤ (1.95 ± 0.30) was detected in 13 lesions; 12 lesions were ependymoma and 1 lesion was pilocystic astrocytoma by histopathology (Fig. 7). Group (3) ADC ratio ≤ (0.93 ± 0.17) was detected in 10 lesions all of them were medulloblastoma by histopathology (Figs. 3, 4, 5, and 6). These results were statistically significant with a P value < 0.001 as shown in Fig. 8 and Table 4.
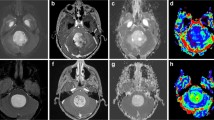
A 5-year-old male patient complaining from headache and vomiting. Axial T1 (a), axial T2 (b), axial FLAIR (c), coronal T1 post-contrast (d), sagittal T1 post contrast (e)—posterior fossa SOL seen filling 4th ventricle and extending through right exit foramina to right cerebellopontine cistern, mass measured 23 × 19 × 44 mm at its maximum AP, width, and CC dimensions showed heterogeneous contrast enhancement (d), (e). Axial DWI (f), axial ADC (g)—the lesion showed restricted diffusion, ADC ratio—1.5, ADC values of the lesion—(1.221, 1.225, 1.226) × 103 mm2/s, average 1.225 × 103 mm2/s, ADC value of the normal cerebellum (0.775, 0.781, 0.785) × 103, average 0.781 × 103 mm2/s consistent with ependymoma but by histopathological examination after surgical excision revealed astrocytoma
ROC analysis for the diagnostic accuracy of ADC ratio of posterior fossa tumors. ADC ratio at a cutoff > 1.7 showed significant good power of discrimination of astrocytoma (AUC = 0.85) from ependymoma with 87.5% sensitivity and 93.3% specificity. Similarly, at cutoff ≤ 1.6-> 1.2 was a significant good predictor of ependymoma (AUC = 0.85) with 87.8% sensitivity and 99.5% specificity. While, ADC ratio ≤ 1.2 was significant excellent discriminator of medulloblastoma (AUC = 0.99) with 100% sensitivity and 90.0% specificity (Table 5) and (Figs. 9, 10, and 11).
Discussion
Diffusion-weighted (DW)-MRI, ADC, and ADC ratio based on tumor cellularity could contribute to the assessment of cerebellar tumors with promising results as regard type and grade [15]. High-grade tumors appear hyperintense on DW images with low ADC values. High tumor cellularity is probably the major factor of the decreased ADC values in high-grade brain tumors [16]. Also, ADC values correlate with total nuclear area and tumor grade with high-grade tumors having high cellular density and decreased ADC [17].
In the current study, we reported that the pediatric posterior fossa tumor more common in females representing 63.32%, their ages ranged from 2 to 17 years with a mean of 8.7 years, which agreed with Ahmed et al. [18], they included 24 pediatric patients (15 females, 9 males) with CNS tumors, they reported that the pediatric posterior fossa tumor more common in females with a mean age of 6 years (range 1 to 15 years). While Zitouni et al. [14] included 42 patients (23 males, 19 females), they reported that the pediatric posterior fossa tumor more common in males with CNS tumors with a mean age of 7.7 years (range 1 to 18 years).
Among studied patients in the current study, ependymoma was the most common pediatric CNS posterior fossa tumor proved by histopathology representing (40%) of the patients followed by medulloblastoma (33.3%) then pilocytic astrocytoma (26.7%). In contrast to the study done by Zitouni et al. [14], it reported that medulloblastoma was the most common pediatric CNS posterior fossa tumor proved by histopathology representing 42.8% followed by JPA then ependymoma. Also, Rumboldt et al. [11] reported that pilocytic astrocytoma was the most common pediatric CNS posterior fossa tumor proved by histopathology representing 48.5% followed by ependymoma then medulloblastoma.
In the current study, ADC values and ratios were significantly different between the commonest three CNS posterior fossa tumors in children: pilocytic astrocytomas, ependymomas, and medulloblastomas (P < 0.001). We reported ADC values were significantly different between pilocytic astrocytomas (1.43 ± 0.28 × 10−3) (mean ± SD) and medulloblastomas (0.71 ± 0. 21 × 10−3) with a P value < 0.001. Also, there was a significant difference when comparing medulloblastomas (0.71 ± 0. 21 × 10−3) with ependymomas (1.04 × 10−3 ± 0.21) with a P value < 0.001.
These results matched with the study done by Zitouni et al. [14], they found a mean ADC values for JPA, ependymoma and medulloblastoma were 1.42 ± 0.27 × 10−3, 1.03 ± 0.21 × 10−3, and 0.707 ± 0.21 × 10−3 mm2/s respectively (P ≤ 0.001). Also agreed with Ahmed et al. [18], they reported ADC values were significantly different between WHO I (low-grade tumors) (1.49056 ± 0.15 × 10−3mm2/s) (mean ± SD) and WHO IV (high-grade tumors) (0.70352 ± 0.23 × 10−3mm2/s), (P ≤ 0.001). Also, Rumboldt et al. [11] reported that ADC values were significantly different between WHO I (low-grade tumors) (1.24 ± 2.09 × 10−3mm2/s) (mean ± SD), ependymoma (0.97 ± 1.29 × 10−3mm2/s), and WHO IV (high-grade tumors) (0.55 ± 0.63 × 10−3mm2/s), (P ≤ 0.001).
In contrast to the study done by Mohamed et al. [19], they reported significant differences in the ADC values between JPA and medulloblastoma also between ependymoma and medulloblastoma (P = 0.000), but no statistically significant differences between JPA and ependymoma (P > 0.05) were detected. There was no overlap in individual tumor ADC values or ratios between JPA and medulloblastoma.
Among the studied patients, ADC ratio was significantly different between pilocytic astrocytomas (1.95 ± 0.30) (mean ± SD) and medulloblastomas (1.01 ± 0.30) with a P value < 0.001, we also demonstrated a significant difference between pilocytic astrocytoma (1.95 ± 0.30) (mean ± SD) and ependymomas (1.50 ± 0.20) (mean ± SD) with a P value <0.001. Also, we found a significant difference when comparing medulloblastomas (1.01 ± 0.30) with ependymomas (1.50 ± 0.20) with a P value < 0.001.
These findings in JPAs and medulloblastomas are probably secondary to the low cellularity and relatively small nuclear area typically seen in the former tumor types in contradistinction to the densely packed cells and large nuclei characteristic for the latter [11].
These results were agreed with Zitouni et al. [14], they found the mean ADC ratios were 1.95 ± 0.30, 1.50 ± 0.20, and 1.02 ± 0.30 for JPA, ependymoma, and medulloblastoma. While Rumboldt et al. [11] found the mean ADC ratios to be 2.11 ± 0.36, 1.39 ± 0.18, and 0.84 ± 0.14 for JPA, ependymoma, and medulloblastoma.
Yamasaki et al. [20] reported that there was a slight overlap in the result between ependymoma and pilocytic astrocytoma. In the present study, we diagnosed two lesions as ependymoma by ADC value but pathologically proved pilocytic astrocytoma. Also, we diagnosed one lesion as ependymoma by ADC ratio but pathologically proved pilocytic astrocytoma.
Finding in ependymoma could be explained by an overlap between ADC values of the classic type (WHO grade 2, one-half of tumors demonstrating restricted diffusion) and anaplastic type (WHO grade 3, two-thirds of tumors demonstrating restricted diffusion), given the wide histologic and prognostic spectrum of ependymoma, diffusion characteristics of ependymoma also have a wide range overlapping other tumor types [21].
We reported the cutoff ADC ratio of > 1.7 for differentiation of pilocytic astrocytomas from medulloblastoma and ependymomas with sensitivity 87.5% and specificity 93.3%. The cutoff ADC ratio of ≤ 1.2 for differentiation of medulloblastomas from PAs and ependymomas is with sensitivity 100% and specificity 90%. The cutoff ADC value of ≤ 1.6, > 1.2 for differentiation of ependymomas from PAs, and medulloblastomas were 100% with sensitivity 87.8% and specificity 99.5%.
These results agreed with Koral et al. [22], they studied 140 patients (58 medulloblastomas, 10 AT/RT, 51 astrocytomas, 21 ependymomas) and could differentiate astrocytoma from ependymoma with 78% sensitivity and 78% specificity using an ADC ratio ≥ 1.8 and could differentiate embryonal tumors from ependymoma with 87% sensitivity and 83% specificity using an ADC ratio ≤ 1.2. Also, Zitouni et al. [14] differentiated astrocytoma from ependymoma with 85.7% sensitivity and 90% specificity using an ADC ratio ≥ 1.7 and differentiated medulloblastoma from ependymoma with 100% sensitivity and 88.89% specificity using an ADC ratio ≤ 1.18, which agreed with our results. Gimi et al. [15] studied 79 patients (31 JPA, 27 medulloblastoma, 14 ependymoma, 7 AT/RT) and measured the ADC ratios as 2.30, 1.58, 0.97, and 0.83 for astrocytoma, ependymoma, medulloblastoma, and AT/RT. They differentiated astrocytoma from ependymoma with 92% sensitivity and 79% specificity using an ADC ratio ≥ 1.7, and differentiated embryonal tumors (medulloblastoma and AT/RT) from ependymoma with 93% sensitivity and 88% specificity using an ADC ratio ≤ 1.2.
Conclusion
ADC ratio is a simple way used in distinguishing juvenile pilocytic astrocytoma, ependymoma, and medulloblastoma, which are the most frequent pediatric posterior fossa tumors. Cutoff ADC ratio of more than 1.7 characteristic of JPA with 87.5% sensitivity and 93.3% specificity, ADC ratio less than 1.1 characteristic of medulloblastoma with 100% sensitivity and 90% specificity. ADC ratios more than 1.1 and less than 1.7 characteristic of ependymoma with 87.8% sensitivity and 99.5% specificity. We recommended ADC ratio as a routine study in the evaluation of pediatric CNS posterior fossa tumors.
Availability of data and materials
The author’s confirm that all data supporting the finding of the study are available within the article and the raw data ad data supporting the findings were generated and available at the corresponding author on request.
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CNS:
-
Central nervous system
- CSF:
-
Cerebrospinal fluid
- CT:
-
Computerized tomography
- DWI:
-
Diffusion-weighted image
- FLAIR:
-
Fluid attenuation inversion recovery
- JPA:
-
Juvenile pilocytic astrocytoma
- MB:
-
Medulloblastoma
- MBs:
-
Medulloblastomas
- MRI:
-
Magnetic resonance imaging
- PA:
-
Pilocytic astrocytoma
- PNET:
-
Primitive neuroectodermal tumor
- ROI:
-
Region of interest
- SOL:
-
Space occupying lesion
- WHO:
-
World Health Organization
- WI:
-
Weighted image
References
Mueller S, Chang S (2009) Pediatric brain tumors: current treatment strategies and future therapeutic approaches. Neurotherapeutics 6(3):570–586
Johnson KJ, Cullen J (2014) Childhood brain tumor epidemiology: a brain tumor epidemiology consortium review. Cancer Epidemiology and Prevention Biomarkers 23(12):2716–2736
Gajjar A, Bowers DC, Karajannis MA et al (2015) Pediatric brain tumors: innovative genomic information is transforming the diagnostic and clinical landscape. J Clin Oncol 33(27):2986–2998
Panigrahy A, Blüml S (2009) Neuroimaging of pediatric brain tumors: from basic to advanced magnetic resonance imaging (MRI). J Child Neurol 24(11):1343–1365
Koob M, Girard N (2014) Cerebral tumors: specific features in children. Diagnostic and interventional imaging 95(10):965–983
Poretti A, Meoded A, Huisman TA (2012) Neuroimaging of pediatric posterior fossa tumors including review of the literature. J Magn Reson Imaging 35(1):32–47
Choudhri AF, Siddiqui A, Klimo P (2016) Pediatric cerebellar tumors: emerging imaging techniques and advances in understanding of genetic features. Magnetic Resonance Imaging Clinics 24(4):811–821
Cheng YC, Lirng JF, Chang FC et al (2005) Neuroradiological findings in atypical teratoid/rhabdoid tumor of the central nervous system. Acta Radiol 46(1):89–96
Gauvain KM, McKinstry RC, Mukherjee P et al (2001) Evaluating pediatric brain tumor cellularity with diffusion-tensor imaging. Am J Roentgenol 177(2):449–454
Brandão LA, Poussaint TY (2017) Posterior fossa tumors. Neuroimaging Clinics 27(1):1–37
Rumboldt Z, Camacho DL, Lake D et al (2006) Apparent diffusion coefficients for differentiation of cerebellar tumors in children. Am J Neuroradiol 27(6):1362–1369
Marupudi NI, Altinok D, Goncalves L et al (2016) Apparent diffusion coefficient mapping in medulloblastoma predicts non-infiltrative surgical planes. Childs Nerv Syst 32(11):2183–2187
Maier E, Sun Y, Mulkern V (2010) Diffusion imaging of brain tumors. NMR Biomed 23(7):849–864
Zitouni S, Koc G, Doganay S et al (2017) Apparent diffusion coefficient in differentiation of pediatric posterior fossa tumors. Jpn J Radiol 35(8):448–453
Gimi B, Cederberg K, Derinkuyu B et al (2012) Utility of apparent diffusion coefficient ratios in distinguishing common pediatric cerebellar tumors. Acad Radiol 19(7):794–800
Mata-Mbemba D, Donnellan J, Krishnan P et al (2018) Imaging features of common pediatric intracranial tumours: a primer for the radiology trainee. Can Assoc Radiol J 69(1):105–117
Bonfield CM, Steinbok P (2015) Pediatric cerebellar astrocytoma: a review. Childs Nerv Syst 31(10):1677–1685
Ahmed HA, Darwish EA, Abo-bakrkhattab OM (2018) Role of diffusion MRI in differentiation between the common pediatric posterior fossa brain tumors. Egyptian Journal of Hospital Medicine 73(2):6090–6096
Mohamed FF, Ismail AA, Hasan DI (2013) The role of apparent diffusion coefficient (ADC) value in the differentiation between the most common pediatric posterior fossa tumors. The Egyptian Journal of Radiology and Nuclear Medicine 44(2):349–355
Yamasaki F, Kurisu K, Satoh K et al (2005) Apparent diffusion coefficient of human brain tumors at MR imaging. Radiology 235:985–991
Jaremko JL, Jans LB, Coleman LT et al (2010) Value and limitations of diffusion-weighted imaging in grading and diagnosis of pediatric posterior fossa tumors. Am J Neuroradiol 31(9):1613–1616
Koral K, Alford R, Choudhury N et al (2014) Applicability of apparent diffusion coefficient ratios in preoperative diagnosis of common pediatric cerebellar tumors across two institutions. Neuroradiology 56(9):781–788
Acknowledgements
To all the participants for their cooperation and patience.
Funding
No funding. Not applicable for this section.
Author information
Authors and Affiliations
Contributions
MD suggested the research idea, ensured the original figures and data in the work, minimized the obstacles to the team of work, correlated the study concept and design, and had the major role in analysis; ME collected data in all stages of manuscript, performed data analysis. EM supervised the study with significant contributions to design the methodology, manuscript revision and preparation. YE correlated the clinical data of patient and matched it with the findings, drafted and revised the work. All authors read and approved the final manuscript for submission.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Informed written consents taken from the patients and healthy volunteers, the study was approved by ethical committee of Tanta university hospital, faculty of medicine.
Committee’s reference number: 32369/6/2018
Consent for publication
All participants included in the research gave written consent to publish the data included in the study.
Competing interests
The authors declare that they have no competing of interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Esa, M.M.M., Mashaly, E.M., El-Sawaf, Y.F. et al. Diagnostic accuracy of apparent diffusion coefficient ratio in distinguishing common pediatric CNS posterior fossa tumors. Egypt J Radiol Nucl Med 51, 76 (2020). https://doi.org/10.1186/s43055-020-00194-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s43055-020-00194-2