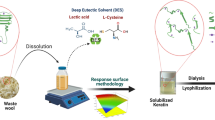
Abstract
Among various protein-containing biomass wastes, waste animal wool, poultry feather, and human hair are considered one of the most important renewable sources of keratin. Animal wool and human hair are utilized for the production of several products. However, the substantial quantity of short fibers that are inappropriate for spinning and being unusable is thrown away as waste resulting in significant environmental issues in terms of their accumulation in water bodies resulting in obstruction of waterways and other related problems. Similarly, poultry wastes, especially waste chicken feathers (WCF) are dumped or burnt or used as low-value fertilizer in certain applications. The purpose of this research is to develop an efficient method that can extract the recoverable keratin from various wastes and effectively utilize the spent solvent in the extraction process. Herein suitability of an aqueous solution of quaternary ammonium hydroxide known as tetramethyl ammonium hydroxide (TMAOH, 25% w/w in water) to solubilize these protein wastes and extract keratin from them was investigated. The solvent could solubilize ca. 39–44% w/w of waste animal wool (WAW), 19–25% of waste human hair (WHH), and 55–60% of WCF. Crude keratin with ca. 19–20%, 35–37%, and 69–74% were isolated from WAW, WHH, and WCF, respectively. The chemical and structural stability of keratin thus isolated was established. The recovered TMAOH, insoluble WAW, and WCF were found to be nontoxic to soil microbes. The recovered TMAOH thus generated after isolation of keratin was used for green gram (Vigna radiata) seed treatment, and a substantial increase in the height (4–12%) and weight (9–58%) of the plants was observed. Treating biomass waste as a source of high-value compounds may minimize environmental impact by reducing the waste load.
Similar content being viewed by others
1 Introduction
Due to the increasing demand using bio-based raw materials for various applications in materials chemistry and engineering, the demand for resourcing such materials is receiving renewed attention from various researchers. To meet the economics and sustainability of any material prepared, the low-cost sourcing of the starting material is of utmost importance. Keratin is one of such proteins, which is the most abundant protein and the major component of hair, feathers, nails, and horns of mammals, reptiles, and birds, and it can be easily extracted from the biomass [1,2,3,4]. Its biodegradability, bio-compatibility, high polarity, high chemical reactivity, and affinity to adhere to cells make it attractive for various applications [5, 6]. The application areas for keratin are tissue engineering, biomedical applications, textile industries, etc. [7,8,9,10,11]. Several functional materials have been designed using keratin as a base in various formats, such as films, fibers, coating, and composite membranes [12,13,14,15]. Keratin is commonly accessible but its extraction from biomass is challenging due to the difficulties in cleaving the protein α-helix and β-sheet due to strong hydrogen bonding and disulfide bonding between polypeptide chains [3, 4, 16, 17]. Although several traditional methods such as reduction, re-oxidation, acid–alkali, enzymatic, and sulfitolysis are being utilized to extract and solubilize keratin from various biomass resources [18,19,20,21], toxicity is associated with several of the solvents and chemicals [22] that pose environmental as well as health concerns [16]. One of the improved methods of extraction of keratin so far reported uses L-cysteine as a reducing agent to extract wool keratin [23].
To bring sustainability to agriculture practices, various reforms in terms of use of better seed quality, deployment of improved technologies towards harvesting and sowing of seeds, etc. are being deployed to increase productivity to cater for the food need of the ever-increasing population of the world [24,25,26]. Seed treatment is considered to be one of the most crucial operations in agriculture, which ensures increased crop yield, quality, and productivity [27]. However, the increasing trend of using pesticides for seed treatment has created an alarming situation in terms of the presence of pesticide residues in seeds and further transport of the harmful molecules to the fruits and flowers, affecting human health. Hence alternative seed treatment solutions are often sought and researched in this area.
Quaternary ammonium electrolytes (QAEs) are emerging as a very good solvent system for the dissolution of biopolymers such as cellulose and certain agricultural wastes [28, 29]. They are believed to resolve the complications such as high viscosity, multiple operations, and the use of high temperature being faced in the dissolution of cellulose [30]. Among the QAEs, it was observed that the anions play a very crucial role in the dissolution process. The poor interaction of the biopolymers (having non-accessible hydroxyl groups due to intra hydrogen bonding) with the QAEs having anions such as chloride and bromide has encouraged the use of QAEs having hydroxyl groups as anion for the effective and efficient dissolution processes [30]. In one of the attempts, we solubilized waste human hair in 40% aqueous solution of tetrabutyl ammonium hydroxide (TBAOH) and isolated keratin and melanin [31].
The purpose of this research is to develop an effective method to extract keratin from various wastes and to effectively utilize the spent solvent in the extraction process. Herein we have demonstrated the suitability of a quaternary ammonium base, namely tetramethyl ammonium hydroxide (TMAOH, 25% w/w in water), for the dissolution of waste animal wool (WAW), waste human hair, and waste chicken feather and isolation of keratin from the solutions. We have further demonstrated the suitability of the recovered solvent for seed treatment similar to the way we have established recently [32]. In the previous study [31], we have considered 40% aqueous solution of TBAOH as an ionic liquid, but because upon evaporation of water, it was not possible to get a molten salt, herein we are calling the solution as a quaternary ammonium base solution rather than ionic liquid. Further, TBAOH was found to be a bit toxic to the soil microbes upon long-term investigation, and hence we have now used TMAOH, which is found to be nontoxic to the soil microbes for seed treatment.
2 Material and methods
2.1 Materials
TMAOH [N(CH3)4+ OH−] (25% w/w in H2O) was purchased from Molychem, Mumbai, India. Synthetic melanin and standard wool keratin were purchased from Sigma Aldrich and TCI respectively. The soil sample was collected from the institute garden (21.7590o N, 72.1443o E), and sludge was collected from a food processing industry waste in Bhavnagar city (21.7515o N, 72.0971o E). The samples were randomly collected and stored in air tight containers. All solvents, such as acetone, HCl, hexane, dichloromethane, etc., used were of AR grade and were used as received from commercial suppliers.
The domestic Indian sheep wool used in these experiments was collected from Bhavnagar's local area, Gujarat, India. At first, the sheep wool was washed three times with water to remove the dust particles. It was cleaned and defatted using a 1:1 v/v mixture of hexane and dichloromethane in a Soxhlet extractor for 48 h. The cleaned wool sample was dried in a vacuum oven at 70 °C for 48 h.
Waste human hair (WHH) was collected from a haircutting salon situated in Bhavnagar City. Human hair samples (50 g) were washed and rinsed thoroughly with 70% (v/v) ethanol and distilled water, followed by soaking in the mixture of chloroform and methanol (2:1 v/v) for 24 h for de-lipidization and air-dried.
The waste chicken feathers (WCF) were collected from local poultry farms situated in Bhavnagar city. The collected feathers were boiled (70 °C) with enough water to wash the material for 3 h while changing the water every one hour and air-dried. Dry feathers were treated with petroleum ether (40–60) [1:20] for 24 h to remove feather lipid. The delipidized feathers were powdered and were used as a starting material.
Green gram seeds of the variety P9072 (Karnal) harvests were obtained from the Indian Agricultural Research Institute, Delhi.
2.2 Dissolution of WAW, WHH, and WCF in aqueous TMAOH
The wool fibers (10 to 400 mg) were gradually added into a vial containing 1.0 mL of an aqueous solution of TMAOH at 65 °C (optimized temperature) for 6 h (optimized time duration) under an atmosphere of nitrogen gas with continuous stirring in glass vials until the fibers were visibly solubilized as shown in Table 1. In addition, a laser beam was used to identify the presence of small particles through the observation of any light scattering from the solutions. In some cases, particularly at high wool contents, the rate of wool dissolution appeared to decrease markedly, probably due to the increased viscosity of the solution. Hence, we describe these ultimate observations as limiting solubility to indicate that these are kinetically limited values rather than thermodynamic solubilities. After 6 h, partial dissolution of wool fibers was observed in TMAOH, and the presence of the insoluble part of fibers was confirmed by observing the aliquots under an optical light microscope (100X). The insoluble part was separated using a centrifuge (8000 rpm for 10 min). The addition of acetic acid and acetone mixture (1:4) in the solution resulted in the precipitation of light yellowish color crude wool keratin as shown in Scheme 1. The keratin was isolated by centrifugation and was washed with acetone (× 5) to remove solvent residues, and placed in a desiccator to obtain dried keratin powder. Keratin thus obtained was sealed and stored at 4 °C before use. A viscous solution was obtained (r-TMAOH) after evaporating acetone present in the solvent mixture remained after the keratin isolation.
The de-lipidized hair fibers (10 to 200 mg) were added progressively into the vial containing 1 mL of TMAOH at room temperature under the atmosphere of nitrogen gas with continuous stirring (up to 9 h). After 9 h, complete dissolution of WHH was observed, and the absence of the insoluble WHH fibers was confirmed by observing the aliquots under an optical light microscope (100X). Black coloured crude melanin was obtained after HCl treatment which was isolated, followed by the addition of acetic acid and acetone mixture (1:4) into the solution, which resulted in the formation of pale brown coloured precipitates (crude keratin). The keratin was isolated by centrifugation followed by washing with acetone, and was placed in desiccators to obtain dried crude keratin powder.
The delipidized chicken feather (10 to 600 mg) were added gradually into a vial containing 1.0 mL of TMAOH at room temperature for 6 h (optimized time duration) under an atmosphere of nitrogen gas with continuous stirring in glass vials until the fiber was observed visually to complete dissolved. In addition, a laser beam was used to identify the presence of small particles through the observation of any light scattering from the solutions. In some cases, particularly at high feather contents, the rate of feather dissolution appeared to decrease markedly, probably due to the increased viscosity of the solution. After 6 h, partial dissolution of chicken feather fibers was observed, and the presence of the insoluble part of fibers was confirmed by observing the aliquots under an optical light microscope (100X). The insoluble part was separated using a centrifuge at 9000 rpm for 10 min. The addition of acetic acid and acetone mixture (1:4) in the solution resulted in the precipitation of light white colour crude feather keratin.
2.3 Cup experiment of green gram
Before performing the germination experiments, green gram seeds were washed thoroughly with 2% sodium hypochlorite solution and sterile Milli-Q water (× 2). A factorial, completely randomized design was used in the present study. The experiment consisted of treating this variety of green gram soaked for 11 min (optimized duration) in the solutions (r-TMAOH). Briefly, each seed was soaked in 10 mL of different r-TMAOH solutions in sterilized test tubes that were ventilated with cotton plugs and then sowed in a cup. The growth of the plant was monitored for 30 d in 4-d of intervals. Water was used for soaking the seeds and was termed a controlled experiment.
2.4 Total microbial count measurement
Microbial growth was monitored in TMAOH, the residue obtained after the dissolution of wool, and recovered TMAOH after the isolation of keratin (r-TMAOH) to ascertain the microbial toxicity and thus the soil compatibility of the samples. The microbes used were isolated from a soil sample collected from the garden area of the institute and an industrial sludge sample collected from Bhavnagar city. In a typical experiment, 500 μL samples were added and plated with agar media. The sludge was diluted to 10–1 and 1 g soil in 10 mL phosphate buffered saline (pH 7) and diluted to 10–2. 100 μL from dilution was spread on Trypticase soy agar plates (duplicates) and incubated at 30 °C for 5 d, and the development of total microbial colony in CFU was monitored after 5 d.
2.5 Characterization
Powder X-ray diffraction (XRD) patterns were obtained at 25 °C using a Philips X'pert MPD System. For each XRD experiment, Cu Kα1 radiation (λ = 1.540 Å) was produced at 40 kV and 30 mA. The data were collected in Brag-Brentano (θ/2θ) horizontal geometry using a 2θ-range of 5 to 80.0° with a step size of 0.02° and an accompanying scan speed of 0.1° s−1. Infrared spectroscopy (FTIR) was performed using a Perkin Elmer, G-FTIR spectrometer (Spectrum GX, GSA). The CHNS analyzer (Elementar, Vario Micro Cube) was used for elemental analyses. 13C NMR spectra were recorded at 400 MHz on a Bruker Avance-400 spectrometer. The 13C CP MAS NMR spectra of these samples were acquired using 10 kHz spinning rate. The contact time in the CP MAS experiments was 2.4 ms with a recycle delay of 1 s and CW decoupling. The number of scans was ∼90 000 to 100,000. The microscopic image of complete dissolution was monitored using a light microscope with 100X magnification (Fine Vision Microscope, India).
3 Results and discussion
3.1 Solubility and extraction
Suitability of aqueous TMAOH to solubilize waste biomass such as animal wool, human hair, and chicken feathers and isolation of keratin are investigated. We previously demonstrated WHH solubility in TBAOH (25% w/w in water) as well as the isolation of keratin and melanin [31]. In addition, we also made certain assumptions that recovered TBAOH might be utilized as a nitrogen fortifier for nitrogen-deficient organic fertilizers. However, further subsequent analysis reveals that TBAOH inhibits the soil microbial development, whereas TMAOH does not (Supplementary Materials, Fig. S1). Hence herein, we have considered studying the suitability of the latter for waste biomass treatment with an aim to use the recovered solvent for seed treatment. It can be observed from Table 1 that ca. 39–44% of WAW, 19–25% of WHH, and 55–60% of WCF are soluble in the quaternary ammonium base solution. It was further noted that the solubility of WHH and WCF was achieved at 25 ± 0.5 °C, while the solubility of WAW was achieved at 65 °C. Solubility was confirmed by observing the aliquots of the solutions under an optical microscope (100X) as described earlier (Figs. S2, S3, and S4) [31]. Maximum keratin was produced from WCF with yield of ca. 70–74%, followed by WHH and WAW. A significant quantity of insoluble material was formed during the dissolution of WAW (ca. 55–60%) and WCF (ca. 40–45%) (Scheme 1).
The lower yield of keratin from WAW is perhaps due to the high-temperature operation which is known to degrade proteins. Furthermore, crude melanin with yield (ca. 20–25%) as identified by UV–Vis spectrophotometer (Fig. S5) could be isolated from human hair. The structure of melanin was confirmed by CP-MAS (Fig. S6) [31]. The insoluble mass obtained from WAW and WCF was further investigated to find their suitability for agriculture applications. In all the cases, the TMAOH utilized was recovered (ca. 30–40%) and was further investigated for soil microbe suitability and seed treatment.
3.2 Chemical and structural stability of keratin
It is necessary to investigate the chemical and structural stability of proteins after dissolution in any solvent to ensure the preservation of the crucial functional behavior of the protein. The FT-IR measurements were done to investigate the change in functional moieties of the protein after dissolution by comparing the spectra with standard wool keratin. The comparison of the spectral bands as shown in Fig. 1 shows characteristic bands due to the peptide bonds (-CONH) and ensured chemical and structural stability of proteins present. It can be seen from the FT-IR spectra of standard natural wool keratin and keratin isolated from all the three waste biomass exhibit identical broad absorption bands of characteristic keratinous fibers between 3350 and 3050 cm−1 corresponding to the N–H and O–H stretching vibration of proteins. The amide I band, which is ascribed to C = O stretching vibration, occurs in the range of 1700 to 1600 cm−1 [33]. An absorption band at 1515 cm−1 was observed due to C–N stretching and N–H bending vibrations (Amide II). The C = O stretching and N–H bending vibrations of Amide-I and amide-II corresponds to the α-helix and β-sheet of the keratin. In Fig. 1, no band is observed in the range of 2550–2600 cm−1, indicating an absence of S–H vibration due to cysteine. The low intensity of the vibration bands at 1083, 1128, and 1161 cm−1 (S–O asymmetric stretching) further confirms the absence of cysteine. In addition, the other bands at 1645, 1402, and 1274 cm−1 correspond to the primary amide, secondary amide, and tertiary amide, respectively. Furthermore, the vibration band observed at 1300–1000 cm−1 is due to the disulfide bonds formed between two alpha-helixes of keratin. The characteristic bands are identical to the one observed for the standard wool keratin indicating preservation of the structural integrity of the protein during extraction in TMAOH.
Since the crystallinity of proteins is very crucial for its functional behavior, it often gets disrupted by the external environment. The crystallinity pattern of the natural wool keratin and keratin isolated from all the three waste biomass using an aqueous solution of TMAOH was investigated by powdered XRD as shown in Fig. 2.
The standard natural wool keratin showed broad diffraction (2θ) peak at ca. 8.7 and 20.0°, corresponding to the α-helix and β-sheet structures of the protein (Fig. 2a) [34]. Similar diffraction patterns corresponding to the α-helix and β-sheet structure of keratin were observed for all the keratin samples extracted from the three waste biomass samples (Fig. 2b-d). However, both the peaks are significantly intense in extracted keratin samples from WAW and WCF, suggesting a higher content of the β-sheet structure in the samples.
13C CP-MAS spectra of the standard natural wool keratin and keratin isolated from all the three waste biomass using aqueous TMAOH are shown in Fig. 3. The spectra showed an asymmetric peak at ca. 173 ppm due to the amide carbonyl carbons of the keratin protein. The peak at 130 ppm is related to the aromatic group-containing amino acids in the keratin. The chemical shifts of α-carbons are recorded between 52 and 56 ppm, while chemical shifts due to the β-carbons in leucine residues and cross-linked cysteine residues are observed at 40 ppm. The carbon peak recorded at 17.5 ppm can be assigned to alanine, and the peak at 22.4 ppm of keratin is due to the β-carbons of leucine. The intense peak centered at 25 ppm can be ascribed to the presence of β-carbons in glutamic acid, glutamine residues, arginine, and cysteine. The signals at lower chemical shifts are due to the alkyl groups of the side chains [13]. The α-carbon peak between 52–56 ppm is broadened in the isolated crude keratin from WAW and WHH, which may be due to the ability of the L-cysteine to disrupt the hydrogen bonding in keratin, leading to the unfolding of the polypeptide chains [13]. This would result in the formation of a greater fraction of β-sheet structures, which is in agreement with the XRD data discussed above.
3.3 Soil compatibility and seed treatment application
As discussed above, a substantial amount of insoluble mass or residue was generated during dissolution, and to utilize them for further applications, they were subjected to elemental analysis. The residue obtained from WAW dissolution was found to have 3.2 ± 0.1% N and 27.4 ± 0.2% carbon, while residue obtained from WCF dissolution was found to have 8.5 ± 0.2% N and 28.5 ± 0.2% carbon. Due to the presence of nitrogen and carbon, they may be treated as good soil nutrients, and hence their soil microbial toxicity was investigated. As shown in Figs. S7 and S8, soil and sludge microbes grow in both the residue samples and the TMAOH solution, indicating their suitability for placing in the soil. It was noted that the pure TMAOH was also nontoxic to the microbes, evident from the formation of microbial colonies of both soil and sludge in the media consisting of the solution (Fig. 4a and b). Moreover, as shown in Fig. 4c and d, 3.2 × 105 CFU g−1 for the soil bacteria were developed in the media consisting of r-TMAOH, while the CFU count was 3.1 × 103 CFU mL−1 for the sludge bacteria (Fig. 4d).
About 30–40% of the processed TMAOH could be recycled (r-TMAOH) as illustrated in Scheme 1. As mentioned above, seed treatment needs some good benign chemicals to enhance the plant quality after germination without affecting human health. Hence use of r-TMAOH for seed treatment is proposed herein. To find the suitability of the recovered solvents for seed treatment, each of the green gram seeds was soaked in 10 mL r-TMAOH/water (1:1) for 11 min and sowed in well-conditioned soil samples under a controlled environment. As can be seen in Figs. S9 and S10, the plant height was increased in comparison to the control treatment (water) by 4%. The weight was increased by 58% for the r-TMAOH treatment obtained from WAW processing after 30 d of sowing, while for r-TMAOH obtained from WCF processing, the plant height and plant weight were significantly increased by 12 and 29%, respectively, after an identical day of sowing. Moreover, after similar days of sowing for r-TMAOH treatment obtained from WHH processing, plant height and plant weight significantly increased by 9%. A representative image showing the increase in the height of green gram plants by r-TMAOH treatment obtained from WAW, WCF, and WHH processing is depicted in Fig. 5.
4 Conclusions and future directions
Herein, a novel and unique strategy for the complete utilization of protein-rich waste biomass such as waste animal wool, human hair, and poultry feathers was demonstrated. The waste biomass was initially solubilized in an aqueous solution of TMAOH (25% w/w in water) followed by isolation of keratin. It was observed that 39–44% w/w of waste animal wool, 19–25% of waste human hair, and 55–60% of a waste chicken feather was solubilized in the solvent. The nitrogen and carbon-rich insoluble mass obtained from the dissolution of animal wool and chicken feather was found to be soil-compatible. Crude keratin with ca. 19–20%, 35–37%, and 69–74% were isolated from waste animal wool, human hair, and chicken feather, respectively. The preservation of the chemical and structural stability of keratin indicated the stability of the protein structure in the solvent system. Normally high temperature is employed to extract protein from waste biomass but herein keratin was isolated from WCF feathers and human hair at room temperature. However, an elevated temperature was required in the case of waste animal wool. The recovered solvent obtained following isolation of keratin was found and shown to be friendly and compatible with soil and sludge microorganisms and was therefore utilized for seed treatment of green gram (Vigna radiata) seeds. After the treatment, a substantial increase in the plant’s height (4–12%) and weight (9–58%) was observed. Considering the easier isolation of protein and maximum utilization of the chemicals, the process demonstrated may be considered as a sustainable method for the production of keratin from waste biomass.
Availability of data and materials
All data generated or analyzed during this study are available upon request.
References
Aluigi A, Corbellini A, Rombaldoni F, Mazzuchetti G. Wool-derived keratin nanofiber membranes for dynamic adsorption of heavy-metal ions from aqueous solutions. Text Res J. 2013;83:1574–86.
Zhang HL, Liu JS. Electrospun poly(lactic-co-glycolic acid)/wool keratin fibrous composite scaffolds potential for bone tissue engineering applications. J Bioact Compat Pol. 2013;28:141–53.
McKittrick J, Chen PY, Bodde SG, Yang W, Novitskaya EE, Meyers MA. The Structure, functions, and mechanical properties of keratin. JOM-US. 2012;64:449–68.
Liu X, Nie Y, Liu YR, Zhang SJ, Skov AL. Screening of ionic liquids for keratin dissolution by means of COSMO-RS and experimental verification. ACS Sustain Chem Eng. 2018;6:17314–22.
Ayutthaya SIN, Tanpichai S, Sangkhun W, Wootthikanokkhan J. Effect of clay content on morphology and processability of electrospun keratin/poly(lactic acid) nanofiber. Int J Biol Macromol. 2016;85:585–95.
DeFrates KG, Moore R, Borgesi J, Lin GW, Mulderig T, Beachley V, et al. Protein-based fiber materials in medicine: a review. Nanomaterials-Basel. 2018;8:457.
Aluigi A, Varesano A, Montarsolo A, Vineis C, Ferrero F, Mazzuchetti G, et al. Electrospinning of keratin/poly(ethylene oxide) blend nanofibers. J Appl Polym Sci. 2007;104:863–70.
Zoccola M, Aluigi A, Patrucco A, Vineis C, Forlini F, Locatelli P, et al. Microwave-assisted chemical-free hydrolysis of wool keratin. Text Res J. 2012;82:2006–18.
Rouse JG, Van Dyke ME. A review of keratin-based biomaterials for biomedical applications. Materials. 2010;3:999–1014.
Silva R, Fabry B, Boccaccini AR. Fibrous protein-based hydrogels for cell encapsulation. Biomaterials. 2014;35:6727–38.
Dickerson MB, Sierra AA, Bedford NM, Lyon WJ, Gruner WE, Mirau PA, et al. Keratin-based antimicrobial textiles, films, and nanofibers. J Mater Chem B. 2013;1:5505–14.
Ruzgar DG, Kurtoglu SA, Bhullar SK. A study on extraction and characterization of keratin films and nanofibers from waste wool fiber. J Nat Fibers. 2020;17:427–36.
Zhao ZY, Song C, Zhou J, Hu RM, Xiao H, Liu YP, et al. An eco-friendly method based on the self-glue effect of keratins for preparing Fe3O4-coated wool. J Appl Polym Sci. 2020;137:49179.
Zhang HL, Ma HR, Zhang R, Wang KR, Liu JS. Construction and characterization of antibacterial PLGA/wool keratin/ornidazole composite membranes for periodontal guided tissue regeneration. J Biomater Appl. 2020;34:1267–81.
Zhong X, Li R, Wang ZH, Wang W, Yu D. Eco-fabrication of antibacterial nanofibrous membrane with high moisture permeability from wasted wool fabrics. Waste Manage. 2020;102:404–11.
Zheng SS, Nie Y, Zhang SJ, Zhang XP, Wang LJ. Highly efficient dissolution of wool keratin by dimethylphosphate ionic liquids. ACS Sustain Chem Eng. 2015;3:2925–32.
Ghanta KP, Pal T, Mondal S, Bandyopadhyay S. Microscopic understanding of the effect of ionic liquid on protein from molecular simulation studies. J Phys Chem B. 2020;124:3909–21.
Yu XJ, Tian JY, Wang JB. Reduction dissolution and cross-linking of wool keratin. Wool Text J. 2012;40:46–9.
Vasconcelos A, Freddi G, Cavaco-Paulo A. Biodegradable materials based on silk fibroin and keratin. Biomacromolecules. 2008;9:1299–305.
Mi X, Li W, Xu HL, Mu BN, Chang Y, Yang YQ. Transferring feather wastes to ductile keratin filaments towards a sustainable poultry industry. Waste Manage. 2020;115:65–73.
Navone L, Moffitt K, Hansen KA, Blinco J, Payne A, Speight R. Closing the textile loop: enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manage. 2020;102:149–60.
Gomez-Herrero E, Tobajas M, Polo A, Rodriguez JJ, Mohedano AF. Toxicity and inhibition assessment of ionic liquids by activated sludge. Ecotox Environ Safe. 2020;187:109836.
Wang K, Li R, Ma JH, Jian YK, Che JN. Extracting keratin from wool by using L-cysteine. Green Chem. 2016;18:476–81.
Pannell DJ. Uncertainty and adoption of sustainable farming systems. In: Babcock BA, Fraser RW, Lekakis JN, editors. Risk management and the environment: agriculture in perspective. Dordrecht: Springer; 2003. p. 67–81.
Guilherme MR, Aouada FA, Fajardo AR, Martins AF, Paulino AT, Davi MFT, et al. Superabsorbent hydrogels based on polysaccharides for application in agriculture as soil conditioner and nutrient carrier: a review. Eur Polym J. 2015;72:365–85.
Scott NR, Chen HD, Cui HX. Nanotechnology applications and implications of agrochemicals toward sustainable agriculture and food systems. J Agr Food Chem. 2018;66:6451–6.
Wimalasekera, R. Role of seed quality in improving crop yields. In: Hakeem K, editor. Crop production and global environmental issues. Cham: Springer; 2015. p. 153–68.
Zhong C, Wang CM, Huang F, Jia HH, Wei P. Wheat straw cellulose dissolution and isolation by tetra-n-butylammonium hydroxide. Carbohyd Polym. 2013;94:38–45.
Zhong C, Wang CM, Wang FX, Jia HH, Wei P, Zhao Y. Application of tetra-n-methylammonium hydroxide on cellulose dissolution and isolation from sugarcane bagasse. Carbohyd Polym. 2016;136:979–87.
Kostag M, Jedvert K, Achtel C, Heinze T, El Seoud OA. Recent advances in solvents for the dissolution, shaping and derivatization of cellulose: quaternary ammonium electrolytes and their solutions in water and molecular solvents. Molecules. 2018;23:511.
Singh N, Prasad K. Multi-tasking hydrated ionic liquids as sustainable media for the processing of waste human hair: a biorefinery approach. Green Chem. 2019;21:3328–33.
Sequeira RA, Pereira MM, Vaghela P, Bhayani A, Dhimmar A, Maru D, et al. Sustainable production of quaternary ammonium seaweed polysaccharide salts and their evaluation for seed dressing in agricultural applications. ACS Agric Sci Technol. 2021;1:674–83.
Kakkar P, Madhan B, Shanmugam G. Extraction and characterization of keratin from bovine hoof: a potential material for biomedical applications. Springerplus. 2014;3:596.
Liu X, Nie Y, SMeng XL, Zhang ZL, Zhang XP, Zhang SJ. DBN-based ionic liquids with high capability for the dissolution of wool keratin. RSC Adv. 2017;7:1981–8.
Acknowledgements
KP, AG, and PV acknowledge CSIR, New Delhi, for financial support for this research (MLP0027). TKM thanks CSIR and Unilever Industries, Mumbai for project fellowships (HCP0019 and CLP1208), and NS thanks UGC for Senior Research Fellowship. PBS and SS acknowledge the Department of Science & Technology for financial support. RS thanks DST for the INSPIRE fellowship. The Analytical and Environmental Science Division and Centralized Instrument Facility of the Institute is acknowledged for providing instrumentation facilities.
Funding
Funding was received from Council of scientific and Industrial Research (CSIR), New Delhi (MLP0027).
Author information
Authors and Affiliations
Contributions
KP has conceived the idea and did overall coordination, planning of the work, writing of manuscript and communicated the manuscript. TKM has carried out all the experimental work and done literature survey. NS has carried out experimental work related to purification of keratin isolated from waste human hair. PV has carried out experimental work related to seed treatment and green gram cup experiments. AG has coordinated and planned experimental work related to seed treatment and green gram cup experiments. SS has carried out experimental work related to isolation of soil microbes and its compatibility with waste solvents. PBS has coordinated and planned experimental work related to isolation of soil microbes and its compatibility with waste solvents. RAS has carried out literature survey and also helped in interpretation of NMR data. The author(s) read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1: Fig. S1.
Soil microbial growth (without dilution) (a) Control medium (b) Medium with added samples of the aqueous solution of tetrabutyl ammonium hydroxide (TBAOH) and tetramethyl ammonium hydroxide (TMAOH). Note: No Bacterial colonies were visible around TBAOH indicating its soil microbial toxicity. However, for TMAOH, microbial colonies were found to form around the sample indicating non-toxicity of the sample to soil microbes. Fig. S2. Microscopic images showing the gradual dissolution of animal wool in 25% aqueous solution of TMAOH. Fig. S3. Microscopic images showing the gradual dissolution of human hair in 25% aqueous solution of TMAOH. Fig. S4. Microscopic images showing the gradual dissolution of the chicken feather in 25% aqueous solution of TMAOH. Fig. S5. UV-Vis spectra of isolated crude melanin from TMAOH. Fig. S6. 13C CP MAS NMR spectra of the (a) standard melanin and (b) isolated crude melanin. Note: The UV-Visible absorbance (200-600 nm) of the extracted crude melanin is shown in Fig. S5. The absorbance spectrum of the melanin from human hair shows a characteristic maximum absorption peak in the UV region at 243 nm and progressively less absorbance in the visible region which is the characteristic of melanin. This is due to the presence of complex conjugated structures in the melanin molecule [1]. All the spectra of melanin pigment generally show strong absorbance in between (200-300 nm) region assigned to the π- π* and n- π* of amino, carboxylic and aromatic moieties [2]. Fig. S7. Microbial growth (soil and sludge microbes) in residue obtained during the dissolution of animal wool in TMAOH. Fig. S8. Microbial growth (soil and sludge microbes) in residue obtained during the dissolution of the chicken feather in TMAOH. Fig. S9. Plant height (cm) of green gram plants (control vs treatments) after 30 d [r-TMAOH obtained from waste (b) animal wool, (c) human hair, and (d) chicken feather processing]. Fig. S10. Plant weight (g) of green gram plants (control vs treatments) after 30 d [r-TMAOH obtained from waste (b) animal wool, (c) human hair, and (d) chicken feather processing].
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Maity, T.K., Singh, N., Vaghela, P. et al. Efficient isolation of keratin from protein-rich waste biomass: a practical approach to minimize environmental impact and valorize waste biomass. Sustain Environ Res 32, 42 (2022). https://doi.org/10.1186/s42834-022-00152-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42834-022-00152-9