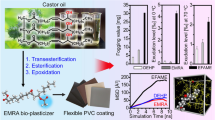
Abstract
Plasticizer migration is responsible for premature coating failure in polyvinyl chloride (PVC) synthetic materials that continue to benefit our daily life as a reliable and cost-efficient simulant of genuine leather. In this context, the establishment of standard assays that measure the migration rate of plasticizers under varying scenarios plays a pivotal role in comparing durability of those PVC-derived leather-simulants. In this review, multiple methodologies developed over the last decade for determining plasticizer migration from PVC coating are compiled, with their operational principles, merits, and limitations being taken into consideration along with specific apparatus required for each. A concluding section discusses current challenges in this field, and highlights how nuclear magnetic resonance and computational simulation surpass conventional assays in yielding intercomparable results, and hence screening migration-resistant plasticizers in a labor- and time-saving way. Since migration resistance represents a decisive performance indicator of plasticizers, this systematic review may provide guidance to quite a few practitioners in PVC synthetic material industry, who are now engaged in validating various sustainable alternatives with performance allegedly equal to conventional but toxic di-(2-ethylhexyl) phthalate plasticizer.
Graphical abstract

Similar content being viewed by others
1 Introduction
Polyvinyl chloride (PVC) synthetic materials, a reliable and cost-efficient simulant of genuine leather, debut in 1930s when the supply of genuine leather failed to meet surging global demand. This leather-simulant is manufactured by applying or laminating a PVC coating, upon adequate finishing, on to one side of a base material that is usually a fabric. After decades of improvement, PVC synthetic materials in contemporary era resemble genuine leather particularly in appearance, and this versatile simulant have been widely used in myriad aspects of our daily life. In 2020, an estimated 2.5 billion square meters of PVC synthetic materials were produced in main-land China, almost five times as much as China’s annual production of genuine leather [1]. Compared with alternatives, the dominance of PVC synthetic materials is largely driven by their excellent processability, mechanical properties, as well as long-lasting durability [2, 3].
To impart the PVC coating with flexibility and bendability, a substantial amount (40–120 wt%) of plasticizers must be added. Plasticizer acts like a lubricant that intercalates the rigid PVC chains for an easy movement, otherwise pristine PVC would be too brittle for any practical purpose. The dosage of plasticizers required in PVC formulation varies according to the end application. In some cases (e.g., upholstery usage), the plasticizer content can be as high as 120 wt% [4]. Since the plasticizers are not chemically bonded to PVC chains, they tend to migrate from the coating during production, storage or service, burdening the environment and shortening the lifespan of the end-products [5, 6]. In particular, di-(2-ethylhexyl) phthalate (DEHP), the most widely used plasticizer now in PVC industry, have been recognized as a human endocrine disruptor, a suspected teratogen, and a probable carcinogen [7]. Their migration raises increasing concerns over the safety issue of PVC synthetic materials [8], driving this old industry into an unprecedented crisis of public confidence and sustainability. Therefore, any emerging plasticizer that succeeds in this arena of intensive competition is required to demonstrate excellent migration resistance, a crucial indicator governing how the resulting PVC coating holds up against long-term service and low environmental impact [9, 10].
In this context, a variety of methods and techniques have been established over the last decade to monitor the migration tendency of plasticizers from PVC coating. Understanding the principle and procedure of these methods is essential for choosing an appropriate approach to evaluate how the plasticizers behave under varying scenarios, and thus providing a reliable criterion to compare durability of the PVC synthetic materials for an intended use. To this end, this review is compiled as a general review of the methods now available for detecting plasticizer migration from PVC synthetic materials via three different mechanisms (i.e., volatilization, exudation, and extraction), with an emphasis on the instrumentation, methodology, advantages, and limitations of each method. In the summary and outlook section, current challenges are briefly discussed, and how recent advances in nuclear magnetic resonance and computational simulation benefit this field by yielding intercomparable results than conventional experimental assays are highlighted. Indeed, a simple literature search for alternatives with performance equal to DEHP but good toxicological profiles unearths a large number of varieties. This systematic review thus may provide important guidance for practitioners on how to evaluate the migration behavior of these sustainable varieties. With potential DEHP alternatives validated, the sustainability challenge now confronting the old and oft-maligned PVC synthetic material industry may ultimately be addressed.
2 Test methods for plasticizer migration
In general, plasticizer migration from PVC synthetic materials can proceed via three different mechanisms, namely volatilization, exudation or extraction, depending on the environment where the PVC coatings are located.
Volatilization refers to diffusion of plasticizers from the center of PVC coating to the surface, followed by evaporation into the environment in high-heat conditions. This can be a continuous process eventually leading to coating embrittlement, as well as environmental and health issues if the PVC synthetic materials are being used in an enclosed space. The plasticizers can also exudate into other materials that are in physical contact with the PVC coating. Conceivably, exudation is facilitated at higher temperature, but can be inhibited by selecting appropriate plasticizers or plasticizer mixtures. In addition, once the PVC synthetic materials are immersed into, or splashed with oil, water or organic solvents, extraction of plasticizers occurs when the liquids dilute away the plasticizer out of the PVC matrix. Extraction usually takes place in combination with PVC swelling that facilitates the movement of additives within, and thus their further diffusion into the liquids.
Taking these three scenarios into full consideration, a couple of standardized methodologies allowing qualitative or quantitative assessment of plasticizer migration have been established over the last decade by organizations such as German Institute for Standardization (DIN), International Organization for Standardization (ISO), and American Society for Testing and Materials (ASTM). These methods have been subject to properly conducted Inter-Laboratory Studies, demonstrating reliability of the test results and statistically determined repeatability. Mechanistically-new test methods are also emerging, and have been successfully used in a few specialized laboratories. In this review, the descriptions of all these methods (Table 1), standardized or emerging, attempt to give some insight into the scientific principles used and the steps that are required to produce a result. Explanations of these methods in terms of their advantages and limitations are also provided to give one a better understanding of their appropriate application.
2.1 Test methods for plasticizer volatilization
Although plasticizers in PVC coatings are relatively nonvolatile at ambient conditions, their migration by volatilization expedites significantly at high temperature [27]. This is particularly the case for a PVC synthetic material-decorated car parked under direct sunlight, or in a plasticizing oven where the gelation temperature is typically between 130 and 170 °C. Currently, the most commonly employed methods that enable quantitative evaluation of the volatilization propensity of plasticizers from PVC synthetic materials are fogging test and activated carbon method.
In automobile industry, fogging refers to heating-induced evaporation of volatile components from PVC, textiles, or genuine leather used in motor vehicle interiors that eventually condense on the windshield to form a haze-like layer. This thin layer of condensate impairs the visibility of the driver, thus endangering the safety of the passengers. In the case of PVC synthetic materials, plasticizers account for the vast majority proportion of the volatile substances.
Until now the German DIN 75201 test method [11] formulated in 1992 has been the ‘gold standard’ to test the evaporation propensity of volatile compounds from vehicle interior materials. Variations of this method include ISO 17071, DIN EN 14288, ISO 6452, and SAE J1756. In DIN 75201, professional apparatus required for the test are specified (Scheme 1), with two methods for determination of the fogging characteristics being provided. In the reflectometric method, a round specimen of PVC synthetic material with a diameter of 80 mm is prepared, with the absorbed water removed by drying over a desiccant. The specimen is placed at the bottom of a glass beaker, the top of which is covered with a glass pane. Throughout the test, the glass pane is thermostated at 21 °C so that any substance evaporated from the specimen can eventually condense on it. Subsequently, the assembly is immersed in an oil bath set to a temperature of 100 °C. In this way, the lengthy evaporation process that normally occurs in motor vehicles under direct sunlight is simulated in ‘quick-motion’. After the heating lasts for 3 h, the glass pane is removed and conditioned, with the amount of fogging condensation being recorded by measuring the 60° reflection value. The fogging value F can then be calculated as the quotient, in percent, of the 60° reflection value for the glass pane with fogging condensate and that of a clean glass pane as a reference. In the gravimetric method, a sheet of aluminium foil is employed to cover the glass beaker containing a specimen at the bottom. After heating the assembly in the oil bath at 100 °C for 16 h, the foil is removed and dried over a desiccant for 4 h before re-weighing. The fogging value G is given by the difference between the masses of the aluminium foil after and before fogging. The results obtained from the reflectometric method and the gravimetric method are not directly related to each other as they measure different parameters.
a Schematic illustration and b photograph [28] of a fogging tester that enables quantitative assessment of plasticizer volatilization from PVC synthetic materials
In comparison, the reflectometric method now is less used than the gravimetric method as the former has been constantly found to produce large inter-laboratory variations. Careful control of the oil bath temperature or cleanliness of the glass pane contributes little to improve the inter-laboratory agreement. The reflectometric method is less reliable, probably because the reflectometer can not discriminate a fog-like, light-scattering condensate on the glass pane and a continuous, transparent plasticizer liquid film displaying little reflection. As such, the fogging value F can look good, even with high amounts of condensate on the glass. In this case, only a combination of gravimetric and reflectometric fogging measurement can guarantee a good overall fogging behavior.
Although a universally recognized method, the fogging test requires tailor-made and expensive apparatus, as well as experienced operators, diminishing its role in industrial settings. Thus, some practitioners refer to follow ISO176:2005(E) [16] that specifies an economically accessible method, intended for a rather rapid comparison of plasticizer volatilization from PVC in the presence of activated carbon. In this method, the PVC synthetic materials are cut into discs of 50 mm ± 1 mm in diameter and 1 mm ± 0.1 mm in thickness. Upon conditioning, one specimen is placed on the bottom of a metal container with its surface being covered by approximately 120 cm3 of activated carbon. Then, two additional specimens are added in the container, each covered by another 120 cm3 activated carbon. Finally, the container is sealed, and incubated at 70 ± 1 °C for 24 h. After that, the specimens are allowed to cool to ambient temperature, and carefully brushed free from any trace of activated carbon before reweighing. Alternatively, contact between the specimens and activated carbon can be avoided by putting each specimen into a small metal wire-mesh cage. In this case, the test temperature should be elevated to 100 ± 1 °C. After 24 h, the specimens are withdrawn from the container, reconditioned and reweighed. Plasticizer volatilization is quantified by the percentage reduction in the mass of the specimens before and after incubation. Although sharing similar underlying principle to the fogging test, the activated carbon method requires neither specific apparatus nor professionally trained operators, facilitating its growing popularity in preliminary screening prior to conduct the fogging test.
2.2 Test methods for plasticizer exudation
Not only do plasticizers evaporate into the air, but also in close contact with solid surface they exudate out of the PVC coatings [29]. Similar to volatilization, plasticizer exudation occurs fairly slowly at ambient conditions so that this process should be evaluated in an artificially accelerated manner. Typical test methods include ISO 177:2016(E) [17] that specifies a standardized procedure to determine the exudation tendency of plasticizers from PVC coatings into other materials that are in physical contact (Scheme 2). In this method, the PVC synthetic materials are cut into discs of 50 mm ± 1 mm in diameter and at least 0.5 mm in thickness. The specimens are then superposed such that the uncoated surfaces come into contact while the surfaces with the PVC coatings point outwards. Upon conditioning, the superposed specimens are placed between two absorbent backing disc, capable of absorbing plasticizers. The absorbent backing discs can be either a standard rubber, an additive-free polyethylene, or a polyvinyl acetate without plasticizer. The sandwich obtained is then placed between two glass plates, followed by being pressed by a flat bottom block of 5 kg and incubated in an oven maintained at 70 ± 2 °C. After 24 h, the specimens are withdrawn and re-conditioned under the same conditions as those applied prior to the incubation. The exudation of plasticizers is quantified by the arithmetic means of the changes in mass of either the specimens or the absorbent backing discs.
Theoretically, the loss in mass of the specimens should be equal to the increase in the mass of the absorbent backing discs. These cases, however, are fairly rare; the observed discrepancy arises due to migration of other constituents other than plasticizers from the PVC coatings. Moreover, some practitioners refer to incubate the specimen sandwich as prepared at 0 °C, instead of 70 ± 2 °C. This variation is carried out in that some plasticizers (e.g., epoxidized fatty acid methyl esters) tend to condensate upon cooling, and thus exudate much faster from PVC matrix than at high temperatures.
Similar procedure that enables measurement of plasticizer exudation are also available in ASTM D2199-03 [18]. In this method, a lacquer film is flattened on a glass panel, and covered by a PVC synthetic material specimen with the PVC coating surface being in close contact with the film. The specimen is then covered sequentially with a foil, a sponge rubber, a glass, and a weight of 910 g, followed by incubation at 50 °C for 72 h. After that, the specimen is withdrawn, the PVC coating surface of which is wiped with a soft rag dampened with heptane. The level of plasticizer exudation can be rated by examining the surface of the lacquer film for any marring or softening. If plasticizer is evident when the specimen surface is wiped, this shall also be reported. Since the test results from ASTM D2199-03 are nonquantitative, it is impossible to specify the precision of the procedures. However, it has been demonstrated that several laboratories assign the same rating to various PVC coating/fabric combinations. Also, replicate tests by one laboratory are in good agreement.
Of course, plasticizer exudation entails no a solid surface that remains close contact with the PVC coating. When a plasticized PVC coating is stressed in compression by bending it through 180°, one way to relieve the stress is by migration of the plasticizer from the compressed area to the area in tension. If these compressive stresses can not be relieved in time by internal migration of plasticizer, the plasticizer will then spew, or ‘sweat’, forming a sticky layer on the coating surface. In general, migration-resistant plasticizers that spew can be re-absorbed rapidly, while those tending to migrate spew early and continue to spew throughout the test. Based on this discrepancy, ASTM D3291-11 [19] defines another standardized procedure that allows qualitative determination of the exudation tendency of plasticizers by rating the amount of plasticizer that spews due to compressional stress. In this method, the conditioned PVC specimens are folded in half, with the short ends together and the loop end pressed in a spacer bar-aided jig (Scheme 3). The assembly is then placed in a chamber at 23 ± 2 °C with a relative humidity of 50 ± 10%. At a fixed time interval, the specimen is removed, bent 360° in the opposite direction, and the former inside of the loop is examined by wiping with a cigarette paper. The likelihood of an exudation can be rated according to the appearance of the resulting cigarette paper, using a scale from 0 to 3 with higher score indicating greater tendency to migrate. A test of seven days duration is usually employed for screening, while an extended test for seven weeks is advised for a complete profile.
Perspective drawing showing specimen mounted in a jig that allows qualitative determination of the exudation tendency of plasticizers from PVC coatings according to ASTM D3291-11 [19]
The apparatus required for this qualitative test is simple and readily accessible, but reproducibility of the test results can sometimes be a problem. Typically, when the specimens prepared from the same origin are no more than 2, multi-laboratory test results are usually within ± 1.0 grading unit at two standard deviations. With a specimen size of 3 or more, practically all tests agree exactly.
Now, imagine a PVC synthetic material containing aromatic hyperbranched polyesters as ‘nonmigrating’ plasticizers. Even at elevated temperature, the polyesters only diffuse to the surface of the coating, forming an ultrathin plasticizer layer of uniform thickness, rather than exudate into other materials being in close contact. As a consequence, the exudation can not be traced by using an analytical balance or any sensory evaluation method. This is particularly the case when heating the specimen in an oven for a long time becomes economically impossible. To address this challenge, Zhang et al. reported a highly sensitive and effective method to examine plasticizer exudation at the molecular level using sum frequency generation vibrational spectroscopy (SFG) [20]. In a typical SFG setup, a mid-infrared beam is sent onto a surface where it is overlapped with a visible beam. Specific properties of the resulting sum-frequency signal (e.g., polarization and intensity) provide information on vibrational properties of the surface with a monolayer sensitivity. Since SFG occurs only if inversion symmetry is broken, it has been reorganized as a specifically surface-sensitive method to distinguish the vibrational fingerprints of the investigated molecules.
In Zhang et al.’s experiment, one PVC coating was put into a clean glass dish, which was sealed with an aluminum foil to prevent potential contamination from the environment (Scheme 4). Even upon heating the assembly at low temperature (30 °C) for a short time (2 h), the characteristic SFG spectrum of phthalate plasticizers on the coating surface could still be detected by using SFG technique. In comparison, the presence of an ultrathin phthalate layer on the PVC coating surface could not be detected by Fourier-transform infrared spectroscopy (FTIR), suggesting the excellent sensitivity of SFG. Zhang et al. estimated that the detection limit by using SFG technique was as low as ~ 30 ng for 1 cm2 PVC coating surface.
Schematic illustration showing specimen arrangement to study phthalate exudation from PVC coating using sum-frequency generation vibrational spectroscopy technique. Reproduced with permission from [20]. Copyright (2014), American Chemical Society
2.3 Test methods for plasticizer extraction
By virtue of excellent abrasion resistance, toughness, and chemical resistance, PVC synthetic materials are now widely used to manufacture suitcase, luggage, flooring, and wallcovering. These products, in their practical application, will be frequently contaminated by liquids (e.g., oil, water, organic solvents, and possibly their mixture), inducing plasticizer extraction. The plasticizers are extractable as they invariably comprise polar and nonpolar domains, rendering them partially compatible with any liquid with a specific polarity.
The simplest and most common method thus far for testing plasticizer extraction has been specified in ASTM D1239-14 [21]. This standardized method proceeds by completely immersing the specimens in a test liquid for a specified time and at a specified temperature, followed by calculating the percentage loss in weight from extraction compared to the original specimen weight (Scheme 5). To cover all typical liquids in household, ASTM D1239-14 suggests the usage of distilled water, soap solution (1%), cottonseed oil, mineral oil United States Pharmacopeia, kerosine and ethyl alcohol (50%) for the test. Acceptable specimen includes a square of 50 ± 0.25 mm on each side, a disk of 50 ± 0.25 mm in diameter, or a tension specimen of 100 by 25 mm as prescribed in ASTM Test Methods D882. Despite the dimension of the specimen, a proportionate amount of test liquid and a container of appropriate size should be used so that the specimen can be immersed in a completely vertical position during the test. To prevent the specimen from floating or curling, it may be necessary to attach small weights. The standard incubation conditions of the immersed specimen shall be 24 h at 23 °C. Alternatively, the incubation can be carried out for 4 h at 23 °C, or either 4 or 24 h at 40 °C. After incubation, the specimen should be gentily wiped with a soft cloth or absorbent tissue before reweighting. If the specimens are immsersed in salt solution, soap, or alkalis, they should be rinsed before wiping to dryness. Here, note that specimens tested in nonvalatile oils (e.g., hexane, heptane, and petroleum ether) require specical attention; they are to be rinsed with a volatile solvent that is a poor solvent for the PVC coating, but a good solvent for the oil before wiping.
Generally speaking, ASTM D1239-14 is an easy-to-operate test that requires no special apparatus. However, chances are, there may be an increase in weight of the specimen at the end of the test if the testing liquid is not volatile and has good adhesion to the PVC coating. In those cases, a weight correction should be determined by conditioning another specimen in the same manner as for the standard test, but immersing it in particular liquid for only 5 min before rinsing and wiping dry. The average percentage weight gain of this blank specimen should be added to the average percentage weight loss of the specimen under test. Meanwhile, the accuracy of the test results from ASTM D1239-14 has also been constantly questioned, as any residual liquid adsorbed on the specimen or the presence of other extractable components results in large experimental deviation. Therefore, some practitioners refer to quantify the extracted plasticizer by measuring the plasticizer concentration in the test liquid using gas chromatography (GC) or liquid chromatography (LC) (Scheme 5). With a calibration curve of corresponding plasticizer in a specific liquid medium, the amount of plasticizer extracted from the PVC coating as a function of incubation time can be accurately measured. Meanwhile, chromatographic technique allows one to discriminate the plasticizer from any other extractable components, significantly improving the accuracy of the test results.
While chromatographic analysis can be automated, conventional soaking extraction is time-consuming, and requires large amounts of solvents. Indeed, laboratories testing plasticizer migration are required to process multiple specimens on a daily basis. As such, the extraction process is often a severe bottleneck in the analytical workflow. In comparison with soaking extraction, accelerated solvent extraction technique provides faster extraction times, reduced solvent use, and automation of the extraction process to benefit laboratories aiming to investigate plasticizer extraction in a highly efficient manner [23, 24]. In this technique, the PVC specimens are grinded into a size of 10 mesh or finer, and extracted by using organic solvents at temperatures above their atmospheric boiling points. The Hildebrand solubility parameter is recommended for choosing an appropriate solvent that reduces the amount of co-extractable materials found in PVC coatings. Since petroleum ether does not appear to solubilize the PVC matrix, it is commonly used under accelerated solvent extraction. However, the pressure exerted on petroleum ether should be elevated to 1500 psi (10 MPa) to ensure the extraction solvent remains in a liquid state at the test temperature (100 °C). Once the extraction completes, the extracts are dried, followed by dissolving the residue in methylene chloride for chromatographic analysis. Comparative experiments demonstrate that accelerated solvent extraction is equivalent to conventional soaking extraction, but requires only 12 min per specimen and approximately 20 mL of extraction solvent, whereas conventional soaking method requires 6 h and 120 mL solvent [30].
3 Summary and outlook
The manufacture of flexible PVC synthetic materials as leather simulant requires addition of a large amount (40–120 wt%) of plasticizers. These additives are supposed to stay in PVC for permanent flexibility, but unfortunately, they continue migrating from the coating matrix, leading to a series of consequences.
A range of analytical methods for the determination of plasticizer migration from PVC synthetic materials is now available. Our review clearly demonstrates that these assay methods differ from one another in terms of apparatus, principles, testing conditions and the way the final results are expressed. Understanding what each test method involves, its benefits and challenges, and how it deviates from one another will help one have a better understanding of how to interpret the data.
Despite the fact that these methods have been validated and standardized, for very practical reasons, many suppliers of PVC synthetic materials or clients are not following precisely the standard procedures, but have introduced their own variations. These variations give rise to incomparability of the obtained results even under framework of the same test methods. For example, despite the popularity of DIN 75201, highly sophisticated methods for measuring plasticizer volatilization from automotive interiors are being requested by some car manufacturers (e.g., Ford-SAE J 1756; GM-GMW 3235; Toyota-TSM 0503 G; Volkswagen-PV 3015; Volvo-VCS 1027, 2719). The principle of these tests is similar to that of DIN 75201, but they are carried out using headspace gas chromatography (HS-GC), or involve small samples (e.g., 10 mg) and varying heating temperatures (85–120 °C). Some methods even specify that the glass pane should be cooled to 38 °C instead of 21 °C. In these cases, variations of the experimental parameters should be present so as to ensure comparability of the measuring results.
Besides, the test methods now available all yield results based on the amount of plasticizers that have left the PVC coatings. This is problematic as the results obtained by different methods are not directly related. Thus, practitioners have to carry out their own tests, even if their clients or suppliers have already submitted the test results, which are usually obtained by undesirable methods. One promising solution to this issue may be measuring the concentration of plasticizers remaining in the PVC coating after the volatilization, exudation, or extraction test. With the residual plasticizer content in the coating known, the results produced by different test methods may be well correlated. Quantification of the plasticizer content in a PVC coating with a thickness of some couples of microns can be done by Fourier transform Raman spectroscopy. The main disadvantage of this method is that it necessitates sample destruction. To address this challenge, in 2015, Alina Adams et al. introduced a simple, fast and nondestructive way to determine the residual concentration of plasticizer in PVC coating by using a portable, single-sided nuclear magnetic resonance (NMR) sensor that collected effective 1H spin–spin relaxation time T2eff and corresponding proton fraction A in steps of 100 μm throughout the coating thickness (Scheme 6a) [25]. By generating a correlation curve between the concentration of plasticizer inside the original PVC coating and the corresponding volume-averaged NMR parameters (i.e., T2eff and A), single-sided NMR allowed quantification of the local concentration of plasticizers in aged PVC coating at different depths by spatially resolved relaxation measurement. Carefully devised experiment validated that this technique was reliable, delivering results in excellent agreement with those obtained from conventional methods (Scheme 6b) [25].
a Schematical illustration of quantifying local concentration of plasticizer in aged PVC coating at different depths using portable, single-sided nuclear magnetic resonance (NMR) sensor. Reproduced with permission from [25]. Copyright (2015), John Wiley & Sons, Inc. b Correlation of the average concentrations of ELATUR CH plasticizer inside aged PVC coating as determined by NMR and gravimetry. The solid line represents the fit result (R = 0.998) using the equation Concentration NMR = − 0.45 + 1.017 × Concentration gravimetry, showing excellent agreement between the data obtained by the two methods. Reproduced with permission from [25]. Copyright (2015), John Wiley & Sons, Inc
Another solution that yields intercomparable results may be computational simulation. Over the past decades, major improvements in simulation speed, accuracy, and accessibility, together with the proliferation of experimental structural data, have increased the appeal of computational simulation to experimentalists. This technique captures the behavior of various molecules in full atomic detail, allowing visualization of micro-scale topographies and dynamic processes at resolutions well beyond the capabilities of experimental means. Inspiring works on using computational simulation to evaluate the migration behavior of plasticizers in PVC coating include those reported by Siyu Pan et al. [22]. To verify whether the strategy they developed was efficient in suppressing plasticizer migration, they reproduced a series of PVC amorphous cells containing a fixed number of different plasticizers (Scheme 7a). Following annealing, the binary cells were equilibrated by using a constant-temperature, constant-pressure ensemble (NPT), from which the mean square displacement (MSD) vs. time plots of the plasticizers were obtained. Self-diffusion coefficients Dα could then be calculated from the slope of the MSD (t) plot according to Einstein relation (Scheme 7b). Good agreement between the calculated Dα and the measured migration trend of the plasticizers under consideration demonstrated the robustness of computational simulation in yielding reliable results. Conceivably, with more migration scenarios established, this technique holds great potential for predicting the migration tendency of different plasticizers without performing tedious and time-consuming experiments. This is highly desirable in PVC synthetic materials industry, since the practitioners are now busy with experiments validating a variety of alternatives with performance allegedly equal to conventional but toxic DEHP.
a PVC amorphous cells containing a fixed number of plasticizer molecules (DEHP: di-(2-ethylhexyl) phthalate; EMO: epoxidized methyl oleate; EB-EMO: ester-branched epoxidized methyl oleate). b MSD of DEHP, EMO and EB-EMO within a well-equilibrated PVC amorphous cell over a 10 ns NPT (P = 1 bar; T = 450 K) dynamics run. Reproduced with permission from [22]. Copyright (2019), The Royal Society of Chemistry
Availability of data and materials
All data generated or analyses during this study are included in this published article.
Change history
06 May 2023
A Correction to this paper has been published: https://doi.org/10.1186/s42825-023-00121-x
References
Global PVC Artificial Leather Market Size, Manufacturers, Supply Chain, Sales Channel and Clients, 2021–2027. https://www.reportsnreports.com/reports/4768692-global-pvc-artificial-leather-market-size-manufacturers-supply-chain-sales-channel-and-clients-2021-2027
Feng G, Hu L, Ma Y, Jia P, Hu Y, Zhang M, et al. An efficient bio-based plasticizer for poly (vinyl chloride) from waste cooking oil and citric acid: synthesis and evaluation in PVC films. J Clean Prod. 2018;189:334–43.
Hou D, Wang S, Chang J, Xu Z, Zeng Q, Wang Z, et al. Cardanol with a covalently attached organophosphate moiety as a halogen-free, intrinsically flame-retardant PVC bio-plasticizer. Fibers Polym. 2020;21(8):1649–56.
McGrath TJ, Poma G, Matsukami H, Malarvannan G, Kajiwara N, Covaci A. Short- and medium-chain chlorinated paraffins in polyvinylchloride and rubber consumer products and toys purchased on the Belgian market. Int J Environ Res Public Health. 2021;18(3):1069.
Erythropel HC, Shipley S, Bormann A, Nicell JA, Maric M, Leask RL. Designing green plasticizers: Influence of molecule geometry and alkyl chain length on the plasticizing effectiveness of diester plasticizers in PVC blends. Polymer. 2016;89:18–27.
Lee KW, Chung JW, Kwak S. Structurally enhanced self-plasticization of poly (vinyl chloride) via click grafting of hyperbranched polyglycerol. Macromol Rapid Commun. 2016;37(24):2045–51.
Suzuki AH, Botelho BG, Oliveira LS, Franca AS. Sustainable synthesis of epoxidized waste cooking oil and its application as a plasticizer for polyvinyl chloride films. Eur Polym J. 2018;99:142–9.
Chen J, Nie X, Jiang J. Synthesis of a novel bio-oil-based hyperbranched ester plasticizer and its effects on poly(vinyl chloride) soft films. ACS Omega. 2020;5(10):5480–6.
Jia P, Zhang M, Hu L, Feng G, Bo C, Zhou Y. Synthesis and application of environmental castor oil based polyol ester plasticizers for poly(vinyl chloride). ACS Sustain Chem Eng. 2015;3:2187–93.
Lee KW, Chung JW, Kwak S. Highly branched polycaprolactone/glycidol copolymeric green plasticizer by one-pot solvent-free polymerization. ACS Sustain Chem Eng. 2018;6:9006–17.
Determination of the fogging characteristics of trim materials in the interior of automobiles. Deutsches Institutfür Normung, DIN 75201:2011.
Leather-Physical and mechanical tests-Determination of fogging characteristics, International Organization for Standardization, ISO 17071:2006.
Leather-Physical and mechanical tests-Determination of fogging characteristics, Deutsches Institutfür Normung, DIN EN 14288:2004–03.
Rubber- or plastics-coated fabrics-Determination of fogging characteristics of trim materials in the interior of automobiles, International Organization for Standardization, ISO 6452:2021.
Determination of the fogging characteristics of interior automotive materials, Society of Automotive Engineers, SAE J1756-2006.
Plastics-Determination of loss of plasticizers-Activated carbon method, International Organization for Standardization, ISO 176:2005.
Plastics-Determination of migration of plasticizers, International Organization for Standardization, ISO 177:2016(E).
Standard test method for measurement of plasticizer migration from vinyl fabrics to lacquers, American Society for Testing and Materials, ASTM D2199-03(2021).
Standard practice for compatibility of plasticizers in poly(Vinyl Chloride) plastics under compression, American Society for Testing and Materials, ASTM D3291-11(2016)e1.
Zhang X, Chen Z. Observing phthalate leaching from plasticized polymer films at the molecular level. Langmuir. 2014;30(17):4933–44.
Standard test method for resistance of plastic films to extraction by chemicals, American Society for Testing and Materials, ASTM D1239-14.
Pan S, Hou D, Chang J, Zhou X, Wang S, Yan S, et al. A potentially general approach to aliphatic ester-derived PVC plasticizers with suppressed migration as sustainable alternatives to DEHP. Green Chem. 2019;21(23):6430–40.
Richter BE, Jones BA, Ezzell JL, Porter NL. Accelerated solvent extraction: a technique for sample preparation. Anal Chem. 1996;68(6):1033–9.
Min N, Yao J, Amde M, Wu L, Sunahara G, Li H, et al. Accelerated solvent extraction combined with GC-MS: A convenient technique for the determination and compound-specific stable isotope analysis of phthalates in mine tailings. Microchem J. 2020;153:104366.
Adams A, Kwamen R, Woldt B, Graß M. Nondestructive quantification of local plasticizer concentration in PVC by 1H NMR relaxometry. Macromol Rapid Commun. 2015;36(24):2171–5.
Pan S, Hou D, Yang G, Xie Q, Yan S, Zeng Q, et al. Epoxidized methyl ricinoleate bio-plasticizer with a pendant acetate ester for PVC artificial material: circumventing existing limit on achievable migration resistance. J Leather Sci Eng. 2019;1(1):1–10.
Ma Y, Liao S, Li Q, Guan Q, Jia P, Zhou Y. Physical and chemical modifications of poly(vinyl chloride) materials to prevent plasticizer migration-Still on the run. React Funct Polym. 2020;147:104458.
Thermo Fisher Scientific-CN. https://www.thermofisher.cn/cn/zh/home.
Zheng T, Wu Z, Xie Q, Fang J, Hu Y, Lu M, et al. Structural modification of waste cooking oil methyl esters as cleaner plasticizer to substitute toxic dioctyl phthalate. J Clean Prod. 2018;186:1021–30.
Kellogg JJ, Wallace ED, Graf TN, Oberlies NH, Cech NB. Conventional and accelerated-solvent extractions of green tea (Camellia sinensis) for metabolomics-based chemometrics. J Pharm Biomed Anal. 2017;145:604–10.
Acknowledgements
We gratefully acknowledge financial support of this work by Engineering Innovation Team Project of Sichuan University (2020SCUNG122), the Opening Project of Key Laboratory of Leather Chemistry and Engineering of Ministry of Education, Sichuan University (SCU2021D005).
Funding
We gratefully acknowledge financial support of this work by Engineering Innovation Team Project of Sichuan University (2020SCUNG122), the Opening Project of Key Laboratory of Leather Chemistry and Engineering of Ministry of Education, Sichuan University (SCU2021D005).
Author information
Authors and Affiliations
Contributions
YC was a major contributor in writing the manuscript. SZ collected and analyzed all relevant literatures. SP was a major contributor in drawing all schemes. DZ, JW, and MZ contributed to this review by discussing advantages and limitations of each test method. HF was responsible for revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chen, Y., Zhou, S., Pan, S. et al. Methods for determination of plasticizer migration from polyvinyl chloride synthetic materials: a mini review. J Leather Sci Eng 4, 8 (2022). https://doi.org/10.1186/s42825-022-00081-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42825-022-00081-8