Abstract
Background
The purpose of this study was to use the MTT test to assess the cytotoxic effects of different extracts of Convolvulus pluricaulis leaves in vitro. Convolvulus pluricaulis leaf ethanolic extract has been demonstrated to trigger apoptosis on HepG2 cancer cell lines, implying anti-cancer activity.
Methods
The cells were grown in culture DMEM and incubated with different concentrations of the plant extracts. Survival rates were quantified by MTT assays by 24 h of exposure to (640–20 µg/ml) the PEE, CHCl3E, EAE, ETHE and AQE of the plant, while monitoring changes on cellular shapes by inverted phase contrast microscopy (PEE—petroleum ether extract, CHCl3—chloroform extract, EAE—ethyl acetate extract, ETHE—ethanol extract, AQE—aqueous extract).
Results
The Convolvulus pluricaulis leaves extract showed IC50 value < 1000 μg/ml on HepG2 and IC50 value > 1000 µg/ml on L-929 cell lines. Hence, Convolvulus pluricaulis leaves extracts are non-toxic against the normal cell line L-929. Treatments with standard as a control exhibited necrotic features in both cell lines. On the basis of these findings, and because a highly effective extract ETHE has a partial polarity, this plant cytotoxicity and apoptotic activities were also investigated. On HepG2 cell line, the ETHE showed higher cytotoxicity activities compared to AQE, EAE, CHCl3E, and PEE extracts (P < 0.0001) with inhibitory concentration IC50 values of 35.873 μg/ml that is < 1000 μg/ml. The percentage of apoptotic cells of ETHE was determined using propidium iodide (PI) staining of DNA fragments by flow cytometry.
Conclusions
The extract of Convolvulus pluricaulis has cytotoxic and apoptotic action against the HepG2 cell line, indicating that it should be studied further for cancer therapies.
Similar content being viewed by others
Background
Hepatocellular carcinoma (HCC) is a type of carcinoma of the liver that arises most commonly in those who have cirrhosis or have had chronic liver illness. Hepatic stem cells are thought to be the source cell(s). According to current knowledge, two primary pathogenic pathways predominate during hepato-carcinogenesis: (i) Cirrhosis is linked to hepatic regeneration after hepatitis infection causes tissue damage; (ii) mutations occurring in single or tumor suppressor genes. Targeting these pathways could assist to reverse, postpone, or prevent tumor development, which is why they are interesting from a therapeutic standpoint (Gaetano et al. 2014). Cancer has been treated with for a long time, Traditional Chinese Medicine, and it may provide some benefits in cancer treatment by decreasing cancer cell proliferation or lowering the negative effects of chemo-radiotherapy (Mcculloch et al. 2011). Several natural compounds have been discovered to have a preference for killing cancer cells while leaving healthy cells unaffected (Chiu et al. 2013). Many epidemiological studies conducted in recent years have suggested that a diet high in vegetables, fruits has anti-carcinogenic and apoptosis-inducing capabilities (Dominguez et al. 2011). Herbal medicines are commonly utilized to treat a wide range of malignancies since they have fewer negative effects than predictable cancer therapeutics (Sahoo et al. 2011). During embryonic progress, immune regulation and tissue homeostasis, apoptosis is the most widely used for programmed cell death mechanism for silently removing unwanted and harmful cells. Furthermore, apoptosis is characterize by the internal proteolytic digestion of the cellular architecture by enzymes known as caspase, resulting in cytoskeletal disintegration, metabolic disruption, and genomic fragmentation (Elmore et al. 2007; Portt et al. 2011; Ouyang et al. 2012). On model cell cultures, the anti-carcinogenic activities of Convolvulus pluricaulis extract have not been completely investigated, and the effects on liver cancer have never been studied. Therefore, this study was conducted to inspect the induction of halting the cell cycle and apoptotic effects of Convolvulus pluricaulis extract on the Liver (HepG2) and Normal (L-929) cell line (Nadia and Amina 2020). The cytotoxic effect of one of these species named Convolvulus pluricaulis was assessed in some cancer cells, and it was unveiled that Convolvulus pluricaulis induces apoptosis in the mentioned cells (Valiyari et al. 2012). Apoptosis is a key cellular process and is a target for development of new anti-cancer therapeutics (Liang et al. 2012). The primary aims of this research include: (i) the determination of the cytotoxic effects of Convolvulus pluricaulis extracts on cancer and normal cells, which have never been investigated; (ii) the precise mechanisms of cytotoxic effects of extracts remain unclear at molecular level. Therefore, study was created to investigate the apoptotic profile of the invading pathogen with PI Annexin V by flow cytometry analysis. To the best of our knowledge, evaluation of the apoptotic effect of Convolvulus pluricaulis has not been evaluated earlier (Nil et al. 2018).
Methods
Plant collection and authentication
The plant materials (leaves) of the Convolvulus pluricaulis were collected by the ‘Prakriti Garden Studio’ in Delhi and authenticated at the National Institute for Traditional Research ICMR, Department of Health, Ministry of Health and family well-being, Government of India, and Belgium RMRC-1447 have been carried out with the access numbers.
Extraction of the plant materials
The fresh leaves plant materials were washed with running tap water and shade dried. The leaves were crushed to coarsely powdered. These coarse powders (45 g) were then subjected to successive extraction in 200 ml of petroleum ether, ethyl acetate, chloroform, ethanol and aqueous solvent by using Soxhlet apparatus. The collected extracts were stored and then used for further analysis.
Phytochemical analysis
Various extracts were tested qualitatively for the presence of phytochemical constituents, namely alkaloids, flavonoids, glycosides, phenols, saponins, sterols, tannins and reducing sugar by following the standard procedure (Deepti et al. 2012).
Cell Lines and cell culture
Two types of cell lines Liver Cell Line-Hep G2 & Normal Cell Line-L929 were purchased from the National Centre for Cell Sciences (NCCS) Pune, maintained in Dulbecco’s Modified Eagle Media (DMEM) with high glucose (Cat No-11965-092) medium supplement with 10% FBS. Cells incubated to a humidified environment, the temperature should be 37 °C containing 5% CO2 (Dingqi et al. 2020).
Antibodies and reagents
Antibiotic:-Solution of Antimycotic 100 × (Thermo Fisher Scientific)–Cat No-15240062. FBS Cat No -10,270,106.
Treatments and experimental design
Tested cell lines were used to assess the cytotoxic effects of different extract of Convolvulus pluricaulis. Cells treated with various concentrations of the prepared extract ranging 640 µg/ml to 20 µg/ml. The cytotoxic activities of different extracts examined were assessed after cell treatment using 3-(4, 5-dimethylthiazol-2-yl), 5-biphenyl tetrazoliumbromide (MTT). Only DMSO was used in the control groups. Furthermore, the morphological changes were evaluated using phase contrast inverted microscope.
MTT cell proliferation assay
For evaluation of cytotoxicity on different extracts of leaves, the IC50 value was determined. The cell line were cultured in Dulbecco’s Modified Eagle Media (DMEM) medium which has been added with 10% heat inactivated fetal calf serum (FBS) and 1% Antibiotic: Anti-mycotic 100 × solution. The cells were seeded at a density of approximately 5 × 103 cells/well in a 96-well flat-bottom micro-plate and maintained at 37 °C in 95% humidity and 5% CO2 for overnight. Different concentration (640, 320, 160, 80, 40, 20 µg/ml) of samples was prepared. For another 24 h, the cells were incubated. After washing the cells twice with phosphate buffer solution, MTT solution (20 µl) for staining (5 mg/ml in phosphate buffer solution) was put in each, and the plate was kept in the incubation chamber at 37 °C. After 4 h, both wells were filled with 100 µl DMSO to make the formazan crystals dissolve. The absorbance was measured, and the results were reported with a 570 nm microplate reader (Stockert et al. 2012).
Formula:
Apoptosis by flow cytometer
The cells were sown in a 24-well flat bottom micro-plate containing cover slips and maintained at 37 °C in CO2 incubator for overnight. The cells were given a half-maximum inhibitory concentration IC50 calculated of treatment ETHE for 12 h. After the incubation, cells were washed with phosphate buffer solution/PBS (pH ~ 7.4) and centrifuged (Remi, CM-12 Plus, India) for 5 min at 500 × g at 4 °C. Discard supernatant and resuspend the cell pellets in ice-cold 1 × Binding Buffer to (1 × 105 per ml) keep tubes in ice-cold. Then add 1 µl solution of Annexin-VFITC and 5 μl propidium iodide (PI) and mix gently. Incubate for 15 min in the dark with the tubes on ice 400 µl of icy cold 1 × binding buffer, lightly mixed. Cell preparations were then processed through a Cytoflex flow-cytometer (Beckman Coulter CA, USA) within 30 min, and assessed with the help of the Cytexpert programme (Version 2.3.0.84) (Bhagwat et al. 2021).
Cell lines and cell culture
Cell lines: Hep G2 (Liver cancer) Dulbecco’s Modified Eagle Media (DMEM) with low glucose—Cat No-11965-092 (Gibco, invitrogen); fetal bovine serum (FBS)—Cat No-10,270,106 (Gibco, invitrogen) Antibiotic–Antimycotic 100 × solution (Thermo Fisher Scientific)—Cat.No-15240062 TACS Annexin V-FITC Apoptosis Detection Kit (R&D Systems) Cat No-4830-01-K.
Statistical analysis
Each experiment was performed at least triplicate and averaged. Values are expressed as mean ± S.E.M (n = 6), data were analyzed using one-way ANOVA, followed by Dunnett’s multiple comparison test by using GraphPad Prism 9 for Windows (disease control vs. treated groups: (*P < 0.05, **P < 0.01, ***P < 0.001)).
Results
The presence of various types of phytoconstituents of the extract was detected by phytochemical screening. The leaves of Convolvulus pluricaulis were found to contain alkaloids, flavonoids, tannins, sterols and carbohydrate.
Cytotoxic activity of Convolvulus pluricaulis leaves extracts on Hep G2 and Line-L-929 cell lines was evaluated by MTT assay. MTT assay is a colorimetric assay that assesses mitochondrial succinate dehydrogenase's reduction of yellow 3-(4, 5 dimethylthiazole-2-yl)2,5-diphenyltetrazolium bromide (MTT). The MTT enters into mitochondria of cell and reduces to an insoluble colored complex (dark purple) formazan. Decline of MTT occurs in the metabolically active cell. The viability of a cell is determined by its amount of activity (Manure and Naikwade 2017).
Effect of extracts with increasing polarity of solvents, namely PEE, CHCl3E, EAE, ETHE, and AQE extracts of Convolvulus pluricaulis on HepG2 & L929 cell line was assessed using MTT assay.
Tables 1, 2 show the IC50 (50% inhibitory concentrations) values of the extracts on the HepG2 & L-929 cell line (Mona et al. 2016).
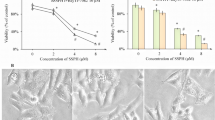
A more meticulous analysis of data unveils that in comparison of Convolvulus pluricaulis of different leaves extract ETHE (IC50 after 24 h: 35.87 μg/ml) in Fig. 1 showed the most potent cytotoxic effects against HepG2 cells followed by the EAE (IC50 after 24 h, 165.67 μg/ml); followed by the AQE (IC50 after 24 h, 419.89 μg/ml); followed by the CHCl3E (IC50 after 24 h, 497.70 μg/ml); then PEE (IC50 after 24 h, 502.70 μg/ml) (Toktam et al. 2016).
In contrast, the extract showed no noticeable cytotoxic effect against L929 as a normal cell line with extremely high IC50 values for these cells: 3294.46 μg/ml for AQE, 2344.90 μg/ml for EAE, 2109.06 μg/ml for ETHE, 2540.20 μg/ml for CHCl3E, 2921.93 μg/ml for PEE (Table 2) (Khulood et al. 2019). Moreover, the cytotoxicity of all the extract on the HepG2 was substantially higher than on L-929 as a normal cell line. However, the IC50 value for Convolvulus pluricaulis extracts was more than 1000 μg/ml on Normal Cell Line-L929, suggesting that this Convolvulus pluricaulis was safe for human consumption. 5-FU was used as a standard or positive control (IC50 after 24 h, 1.66 μg/ml). The characteristics of 5-FU were toxic at cell levels showing lowest survival rate among other treatments in a dose-dependent manner (Fig. 2).
Images of different extracts from Convolvulus pluricaulis treated with HepG2 cells indicate viable cells (VI), chromatin condensation (CC), membrane blabbing (BL), apoptotic bodies (AB) and dead cells (DC); darkened stains indicating DNA fragmentation within the cells.
The control cells, however, remained intact and evenly shaped. When treated for 24 h, most cells remained intact but lost their shape. Following treatment with ETHE of Convolvulus pluricaulis, all cells were completely ruptured see in Fig. 3. The staining noticeably showed apoptotic morphological changes in ETHE of Convolvulus pluricaulis treated cells in terms of both nuclear condensation and cell structure loss.
The control cells displayed a bright reddish brown coloration and an intact nuclear structure, indicating healthy viable cells, whereas treated cells exhibited bright red stain signifying apoptotic cells are present.
Fibroblast cells of the normal cell line L929 were flat, elongated and spindle shaped (aligned in parallel cluster). There were no morphological changes in the cells when different extracts of Convolvulus pluricaulis were applied at varied concentrations shown in Fig. 4. The cell proliferation rate and untreated L-929 cell growth were both satisfactory. So Normal Cell Line-L929 is biocompatible with extracts of Convolvulus pluricaulis (Fig. 5).
Flow-cytometric analysis of (A) Control and (B) Convolvulus pluricaulis of ETHE and Annexin V-FITC & PI double labeling were used to trigger apoptosis in Hep G2 cells. Results represent both panels indicate (Annexin +/PI −) early apoptosis in lower right quadrant/ (Q3), while (Annexin + /PI +) late apoptosis in the upper right quadrant/(Q2). The quadrant on lower left represents viable cells/(Q4). The upper left quadrant shows necrosis (Annexin −/PI +) (Q1)
Convolvulus pluricaulis extracts tested in the present study had lower IC50 value suggesting higher level of cytotoxicity activity against HepG2 cells compared to L-929 cell (Anith et al. 2020).
Annexin V-FITC assay of Convolvulus pluricaulis of ETHE treated HepG2 cells
The cells were considered apoptotic if they were positive for Annexin-V and negative for propidium iodide. When the cells were positive for both the Annexin-V and propidium iodide, however cells that had been declared to be dead. Similarly, when the cells were negative for both the Annexin-V and propidium iodide they were identified as viable cells (Nael et al. 2015).
Annexin V-FITC analysis of HepG2 cells Control (A) and treated with Convolvulus pluricaulis of ETHE for 12 h. (B). The Annexin V assay revealed that Convolvulus pluricaulis of ETHE induced early and late stage of apoptosis in HepG2 cells. The Annexin V-FITC plots in Fig. 6 show the HepG2 cell distribution within four different quadrants (Q1, Q2, Q3, and Q4).When compared with untreated cells, treated cells had a lower percentage of viable cells. The percentage of viable cells decreased from 98.8% to 70.00% after 12 h of treatment.
In the untreated cells, there was minimal cell distribution in Q1, Q2 and Q3 indicating a very low number of dead, early and late apoptotic cells, respectively. However, the cell distribution in these quadrants increased after treatment with ETHE.
The percentage of early apoptotic cells (Q3) remains the same in untreated cells and treated cells (0.00%). The untreated HepG2 cells showed a distribution of 0.81% for the late apoptotic cells (Q2).
Similar to viable cells, the late apoptotic cells percentage of in untreated HepG2 cells (0.81%) increased to (15.2%) after 12 h of ETHE treatment, respectively. The raise in cell distribution indicated a time-dependent increase in late apoptotic cells.
Lastly, dead cells, represented by (Q1) the ETHE treatment, there was only a small increase in cell dispersion. For a 12-h incubation period, the ETHE treated cells had a percentage of dead cells of 0.38 percent, while the untreated cells had a percentage of dead cells of 14.8 percent (Fig. 5 and Table 3). ETHE generated a considerable increase in apoptosis in HepG2 cells, as seen by the total cell population shift (Saie et al. 2018).
Discussion
Hepatocellular carcinoma (HCC) is a kind of cancer which affects the liver. It is one of the most common tumors in the world, accounting for over 90% of all primary liver malignancies (Zhao et al. 2018). Therefore, there is essential to discover new chemo preventive agents that are effective and have no side effects for growth inhibition of hepatocellular carcinoma. The HepG2 cell line has been used widely to study liver cancer (Chandrasekaran et al. 2010).
These medicinal plants have various biological properties, including, antitumor activity, headache treatment, antidepressant, anti-inflammatory, antioxidant, effect on learning and memory, Anti-microbial, insecticidal, anti-fungal, anti-bacterial and anthelmintic activity and anti-diabetic activities (Satish et al. 2014).
In our study, MTT assay is such a technique; this has the potential to have a huge impact in preclinical cytotoxicity screening. The reduction of tetrazolium salt by enzymes, the MTT assay determines the functional integrity of mitochondria and offers information on cellular metabolism and cell viability (Manure and Naikwade 2017).
The cytotoxicity of two cell lines, Hep G2 and Line-L929, was assessed using the MTT assay. The IC50 value was obtained to assess its inhibitory concentration that causes 50% cell viability (Damita et al. 2020). Using cytotoxic characteristics as a criterion of Hep G2 & Line-L929 cell lines, it was observed that there was decreased cell viability in tandem with increasing concentration and time of treatment (Maryam et al. 2020). Cancer cells' resistance to apoptosis is one of its distinguishing features (Mei-lan HE 2007; (Mense et al. 2008).
As a result, a thorough knowledge of the apoptotic signaling pathways involved is critical for the identification and development of target-specific therapies. Carcinogens can be studied using mouse models. They will substantially contribute to understanding of cancer causation and molecular pathways (Jiang and Yu 2017; Dunn and Umar 2016).
Therefore, the current study using HepG2 cells as in vitro models aims to provide insights into the cell death mechanism and molecular action mechanism against apoptosis by its bioactive compounds.
The plasma membrane's phospholipids are asymmetrically distributed across the inner and outer leaflets. The lipid bilayer's external leaflet exposes phosphatidylcholine and sphingomyelin, while the inner leaflet exposes phosphatidylserine. This asymmetry is disturbed during apoptosis, and phosphatidylserine is exposed on the plasma membranes outside surface (Fadok et al. 1992; Koopman et al. 1994; Van Engeland et al. 1998).
A decrease in viability was observed at 24 h when the cells are incubated along with ETHE, indicating that ETHE shows an inhibitory trend on HepG2 cell growth by significant accumulation of cells in the Sub-G0 phase.
These results indicated that treatment with ETHE resulted in apoptotic cell death and Sub-G0 phase cell cycle arrest, thereby delaying the progression of the cell through the G1, S and G2/M phase in HepG2 cells (Chen et al. 2015).
One of the markers for predicting cell membrane injury is the level of LDH in the culture medium. ETHE stimulated the release of LDH in HepG2 cells, as expected. These findings strongly suggest that ETHE inhibits HepG2 cell growth, probably due to rupture of the cell membrane, which results in cell death.
These findings strongly suggest that ETHE inhibits HepG2 cell growth, probably due to rupture of the cell membrane, which results in cell death (Chen et al. 2015).
Interestingly, the ETHE exhibited a higher selectivity than the PEE, CHCl3E, EAE and showed significant cytotoxicity and a high selectivity against HepG2 cells after 24 h exposure (Sasipawan et al. 2012).
Conclusions
This article focuses on a study of the cytotoxicity and apoptotic potential of ETHE of Convolvulus pluricaulis on HepG2 cells. In further studies using Annexin V-FITC analyses established significant increases in late and dead apoptosis. The ETHE of Convolvulus pluricaulis caused cell membrane integrity damage, and initiated an apoptotic response in HepG2 cells. These results suggest that this ETHE of Convolvulus pluricaulis is a good candidate for the discovery of novel cancer medicines in humans. These findings will contribute to the effort to identify phytochemicals that can potentially function as human anticancer agents. Our results clearly show a selective cytotoxicity and apoptosis inductive effect indicative of an anti-cancer activity of the ETHE of Convolvulus pluricaulis on the human hepatoma HepG2 cell line. Further, the present study concludes that different extracts of leaves of Convolvulus pluricaulis were sufficiently safe for human consumption. The anti-cancer capabilities of Convolvulus pluricaulis need to be further investigated in different cancer types and stages, exploiting the full potential as new alternatives in pharmacological and medicinal applications.
Availability of data and material
Not applicable.
Abbreviations
- AQE:
-
Aqueous extract
- EAE:
-
Ethyl acetate extract
- ETHE:
-
Ethanol extract
- CHCl3E:
-
Chloroform extract
- PEE:
-
Petroleum ether extract
- C. pluricaulis :
-
Convolvulus pluricaulis
- MTT:
-
3-(4,5-Dimethylthiazol-2-yl), 5-biphenyl tetrazolium bromide
- Hep G2 (or HepG2):
-
Human liver cancer cell line
- 5-FU:
-
Fluorouracil
- FBS:
-
Fetal bovine serum
References
Alonso-Castro AJ et al (2011) Mexican medicinal plants used for cancer treatment: pharmacological. Phytochem Ethnobot Stud J Ethnopharm 133(3):945–972
Anith MM et al (2020) Cytotoxicity and anticancer activity of Donkioporiella melleaon MRC5 (Normal Human Lung) and A549 (Human Lung Carcinoma) cells lines. Evidence-based Complem Altern Med 7415672:1–10
Baliga MS, Dsouza JJ (2011) Amla (Emblica officinalis Gaertn), a wonder berry in the treatment and prevention of cancer. Eur J Cancer Prev 20(3):225–239
Bertino G et al (2014) Hepatocellular carcinoma: novel molecular targets in carcinogenesis for future therapies. Biomed Res Int 2014:203693
Bhagwat DA et al (2021) Capsaicin loaded solid SNEDDS for enhanced bioavailability and anticancer activity: In-Vitro, In-Silico, and In-Vivo characterization. J Pharm Sci 110(1):280–91
Chandrasekaran K et al (2010) Apoptosis in HepG2 cells exposed to high glucose. Toxicology in-Vitro 24(2):387–396
Chen J et al (2015) Enhancing effect of natural borneol on the cellular uptake of demethoxycurcumin and their combined induction of G2/M arrest in HepG2 cells via ROS generation. J Funct Foods 17:103–114
Chiu CC, Haung JW, Chang FR et al (2013) Golden berry-derived 4 beta-hydroxy with anolide E for selectively killing oral cancer cells by generating ROS, DNA damage, and apoptotic pathways. PLoS ONE 8:E64739
Damita LC et al (2020) Assessment of in-vitro biological activities of Terminalia arjuna Roxb. bark extract and Arjunarishta in inflammatory bowel disease and colorectal cancer. Indian J Exp Biol 58:306–313
Deepti KPU, Vijayalakshmi G (2012) Antimicrobial activity and phytochemical analysis of Morinda tinctoria Roxb. Leaf extracts. Asian Pac J Trop Biomed 2:1440–1442
Dingqi S et al (2020) MK2206 enhances cisplatin-induced cytotoxicity and apoptosis in testicular cancer through akt signaling pathway inhibition. Transl Oncol 13:100769
Dominguez M et al (2011) Anti-inflammatory activity of Penstemon gentianoides and Penstemon campanulatus. Pharm Biol 49(2):118–124
Dunn BK, Umar A (2016) Richmond, E. Introduction: cancer chemoprevention and its context. Semin Oncol 43:19–21
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Fadok VA et al (1992) Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol 148:22–29
Gaetano B et al (2014) Hepatocellular carcinoma: novel molecular targets in carcinogenesis for future therapies. Biomed Res Int. 2014:203693
Jiang Y, Yu Y (2017) Transgenic and gene knockout mice in gastric cancer research. Oncotarge 8:36–96
Khulood MA et al (2019) Selective cytotoxic effect of Plantago lanceolata L. against breast cancer cells. J Egypt Natl Cancer Inst 31–10:2–7
Koopman G et al (1994) Annexin V for flow cytometric detection of phosphatidyl serine expression of B cells undergoing apoptosis. Blood 84:1415–1420
Liang CZ et al (2012) Matrine induces caspase-dependent apoptosis in human osteosarcoma cells in-vitro and in-vivo through the up-regulation of Bax and Fas/Fas L and down regulation of Bcl-2. Cancer Chemother Pharmacol 69(2):317–331
Manure JY, Naikwade NS (2017) Evaluation of anticancer activity of leaves of Rumex vesicarius Linn and Symplocos racemosa Roxb. by brine shrimp lethality and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) method. Int J Green Pharm 11(4):S749
Manure JY, Naikwade NS (2017) Evaluation of anticancer activity of leaves of Rumex vesicarius Linn and Symplocos racemosa Roxb. by brine shrimp lethality and (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) methods. Int J Green Pharm 11(4):749
Maryam S et al (2020) In-vitro and in-vivo anticancer activity of the mostcytotoxic fraction of Pistachio Hull extract in breast cancer. Molecules 25:1776
Mcculloch M, Broffman M, Van der Laan M et al (2011) Lung cancer survival with herbal medicine and vitamins in a whole-systems approach: ten-year follow-up data analyzed with marginal structural models and propensity score methods. Integr Cancer Ther 10:260–279
Mei-lan HE (2007) Mechanisms of anti-prostate cancer by gum mastic: NF-B signal as target. Acta Pharmacol Sin 28:446–452
Mense SM et al (2008) Phytoestrogens and breast cancer prevention: possible mechanisms of action. Environ Health Perspect 116:426–433
Mona O et al (2016) Cytotoxic and apoptotic activities of methanolic subfractions of Scrophularia oxysepa against human breast cancer cell line. Science 8540640:1–10
Nadia FH, Amina FF (2020) Anticancer effects induced by artichoke extract in oral squamous carcinoma cell lines. J Egypt Natl Cancer Inst 32:17
Nael A et al (2015) Apoptotic potential of Artemsia sieberia Besser (Asteraceae) fraction against human cancer cell lines. Trop J Pharm Res 14(10):1779–1785
Nil K et al (2018) Evaluation of in-vitro anticancer activity of vulpinic acid and its apoptotic potential using gene expression and protein analysis, Indian. J Pharm Educ Res 52(4):626–634
Ouyang L, Shi Z, Zhao S et al (2012) Programmed cell death pathways in cancer: a review of apoptosis, autophagy and programmed necrosis. Cell Prolif 45(6):487–498
Portt L et al (2011) Anti-apoptosis and cell survival: a review. Biochimicaet Biophysica Acta Mol Cell Res 1:238–259
Sahoo N et al (2011) Herbal drug patenting in India: IP potential. J Ethnobiol Ethnomed 137(1):289–297
Saie BK et al (2018) Induction of apoptosis and cytotoxicity by isothiocyanate sulforaphene in human hepatocarcinoma HepG2 cells. Nutrients 10:718
Sasipawan M et al (2012) Anticancer effect of the extracts from Polyalthia evecta against human hepatoma cell line (HepG2). Asian Pac J Trop Biomed. 2(5):368–374
Satish AB et al (2014) Ethnobotany, Phytochemistry and Pharmacology of Convolvulus pluricaulis, Choisy. Res J Pharm Biol Chem Sci 5(3):629–636
Stockert JC et al (2012) MTT assay for cell viability: Intracellular localization of the formazan product is in lipid droplets. Acta Histochem 114(8):785–796
Toktam M et al (2016) Oncology letters evaluation of the cytotoxic effects of Cyperus longus extract, fractions and its essential oil on the Pc3 and Mcf7 cancer cell lines. Science 11:1353–1360
Valiyari S et al (2012) Dichloromethane and methanol extracts of Scrophularia oxysepala induces apoptosis in MCF-7 human breast cancer cells. Adv Pharm Bull 2(2):223–231
Van Engeland M et al (1998) Annexin V-affinity assay: a review on an apoptosis detection system based on phosphatidylserine exposure. Cytomet J Int Soc Anal Cytol 31(1):1–9
Zhao Y et al (2018) A. Apoptosis and autophagy induction of seleno-β-lactoglobulin (Se-β-Lg) on hepatocellular carcinoma cells lines. J Funct Foods 49:412–423
Acknowledgements
The authors are grateful to the administrators of Dr. Prabhakar Kore Basic Science Research Center, KLE University, Belagavi, Karnataka, India, for allowing them to conduct cell culture experiments in their laboratory.
Funding
Not applicable. This research received no specific support from governmental or private funding organizations.
Author information
Authors and Affiliations
Contributions
A.M.T. gave a brief idea of the project and took efforts in conducting the study. K.A.W. carried out the cytotoxicity assay studies also contributed to the study's design and completed the statistical analysis, as well as helping to draught the report. The final manuscript was read and approved by all writers. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethical approval and consent to participate.
Not applicable: This article does not contain any studies with human or animal subjects, only use Two types of cell lines: Liver Cell Line-HepG2 and Normal Cell Line-L929 were purchased from the National Centre for Cell Sciences (NCCS) Pune, maintained in Dulbecco’s Modified Eagle Media (DMEM) with high glucose (Cat No-11965-092) medium supplemented with 10% FBS. The cells were incubated in a humidified environment at 37 ºC containing 5% CO2. The research did not require any approvals because it followed all applicable regulations.
Consent for publication
Not applicable.
Competing interests
There are no competing interests declared by the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tamboli, A.M., Wadkar, K.A. Comparative cytotoxic activity of Convolvulus pluricaulis against human hepatoma cell line (HepG2) and normal cell line (L929) via apoptosis pathways by flow cytometry analysis. Bull Natl Res Cent 46, 145 (2022). https://doi.org/10.1186/s42269-022-00835-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-022-00835-8