Abstract
Background
Economics and human safety to avoid health risks caused by fungicides are materializing new era of biological pest control. Trichoderma species ranked high among other agents to control complex black root rot disease of strawberry caused by Fusarium solani, Rhizoctonia solani, and Pythium sp. Our study aimed to document the efficacy of local strains representing T. harzianum, T. viride, T. virinis, and T. koningii against such a disease.
Materials/methods
These strains were cultured separately on potato dextrose broth medium to test their inhibitory effect against strawberry black root rot in vitro and in vivo. Strawberry growth and yield were also assessed relative to the untreated check and the fungicide Actamyl. Activity of peroxidase and chitinase were measured in plant leaves using spectrophotometer.
Results
Each of the antagonistic fungal strains significantly reduced growth area of all pathogenic fungi collectively causing the disease. Trichoderma harzianum, T. viride, and T. koningii reduced the growth area more than 90.6% for all tested pathogenic fungi. Each species significantly reduced disease incidence and severity under field conditions. The highest reduction in the disease incidence and severity, 83.3 and 88.5% respectively, was attained by mixture of the four species. This mixture increased the strawberry fresh and dry weight by 83.3 and 176.9%, respectively, and the yield by 117.1%. All Trichoderma species tested significantly increased the activity of two plant defense-related enzymes of strawberry plants against the pathogens. Their mixture attained the highest increase of peroxidase and chitinase activity by 150 and 160.9%, respectively.
Conclusions
While the fungal mixture could considerably increase the strawberry fresh and dry weight as well as the yield, it suppressed the incidence and severity of the disease. So, integrated pest management in ways that make these biocontrol agents complementary or superior to chemical fungicides should further be examined against this disease.
Similar content being viewed by others
Background
Black root rot is a complex disease because several fungi usually work together giving rise to its occurrence (Fang et al. 2012; Hutton et al. 2013; Juber et al. 2014). This complex disease is characterized by feeder rootlet killing, deterioration and blackening of the main root system, and a decline in vigor and productivity of the plant stand causing considerable reduction in the yield (Abdet-Sattar et al. 2008; Ceja-Torres et al. 2014).
Biological control agents rank high among other fungal management options given mounting care to lessen application of chemical fungicides with a clear aim at the avoidance of human health hazards and attaining pollution-free environment. Trichoderma spp. can be considered as ideal biocontrol agents for their good characteristics. Using T. harzianum and T. viride as seedling dressing to suppress root rot of strawberry and improve its yield components has long been tried (Sullivan 2004). Both fungi showed dual effect by producing growth regulators (Karlidag et al. 2012) and antioxidant effect, thus improving plant physiology and metabolism (Hernandez et al. 2011).
Ahmed and El-Fiki (2017) reported that T. harzianum, T. album, T. viride, and plant guard were highly effective antagonists as shown by supporting strawberry plant survival against root rot disease. So, those treatments scored best fruit yields under field conditions. Moreover, application of these antagonists recorded the highest increase in total phenols, total nitrogen percentage, and total chlorophyll of strawberry in comparison with the untreated check. The mechanisms of Trichoderma action in plant protection have been recorded. For example, T. harzianum secretes many lytic enzymes like chitinase, glucanases, and proteases which help parasitism of plant pathogens. The chitin layer of the pathogen is dissolved via enzymatic activity. The hyphae of T. harzianum penetrate the pathogen, proliferate within the organism, and produce toxic metabolites. Likewise, T. viride produces antibiotics like trichodermin, dermadin, trichoviridin, and sesquiterpene heptalic acid which are involved in the suppression of the pathogens (Abd-Elgawad and Askary 2018).
Trichoderma album, T. hamatum, T. harzianum, and T. viride were reported to significantly reduce the mycelial growth of Fusarium solani and Rhizoctonia solani. In this respect, the activity of plant defense-related enzymes against pathogens such as chitinase, peroxidase, and polyphenol oxidase was increased in treated strawberry plants compared to untreated ones (Cherif et al. 2007; Latha et al. 2009; Prlak and Kose 2009; Saksirirat et al. 2009).
Currently, Egypt is experiencing a revival of the strawberry cultivation backed by its Mediterranean climate, fertile soils, and geographic location, which support high production and profitability of such a specialty crop. These factors can collectively offer early fruiting and long harvest season, good quality, low production costs, and closeness of export markets (Abd-Elgawad 2019). However, a few pests and diseases can cause considerable yield losses in the crop size and quality. Therefore, updated comprehensive revisions to control strawberry white grub or rootgrub Temnorhynchus baal (Shehata et al. 2019), strawberry leaf blight disease caused by Phomopsis obscurans (Abd-El-Kareem et al. 2019a), and plant-parasitic nematodes (Abd-Elgawad 2019) are in progress. Field application and commercialization of such biocontrol agents are being investigated in Egypt and worldwide (e.g., Ziedan et al. 2011 and Ziedan et al., 2015; El-Mougy and Abdel-Kader 2014; Abd-Elgawad and Vagelas 2015; Abd-Elgawad 2017; Abd-Elgawad and Askary 2018). Yet, little attention is given to black root rot of strawberry which ranks high among strawberry diseases (El-Shemy et al. 2013). The aim of this study is to document the effects of some local Trichoderma spp. as safe biocontrol agents against strawberry black root rot under field conditions in Egypt. Also, some of the species herein have not been tested against the disease yet.
Materials and methods
Pathogens
Local pathogenic isolates of F. solani, R. solani, and Pythium sp., the causal agents of black root rot disease of strawberry plants, in addition to four antagonistic species of Trichoderma, i.e., T. harzianum, T. viride, T. virinis, and T. koningii were identified and provided by the Plant Pathology Department, National Research Centre, Giza, Egypt (Elshahawy et al. 2017; Abd-El-Kareem et al. 2019b).
Plant material and inoculum preparation
Strawberry seedlings (cv. Festival) were obtained from the Vegetable Crops Research Department, Agricultural Research Centre, Giza, Egypt. Inocula of antagonistic fungi were prepared by culturing each isolate on 50 ml potato dextrose broth (PDB) medium in 250-ml Erlenmeyer flasks for 15 days at 25 ± 2 °C. Inoculum of each fungus was prepared from its growing upper solid layers which were washed and blended in sterilized water. Colony-forming units (cfu) were adjusted to 106 cfu/ml using hemocytometer slide (Harman et al. 2004). A few drops of the emulsifier Tween 20 (Sigma Co.) and sticker were added.
Laboratory test
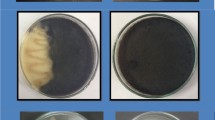
Trichoderma harzianum, T. viride, T. virinis, and T. koningii were tested for their inhibitory effect against strawberry black root rot fungi, i.e., R. solani, F. solani, and Pythium sp. in vitro. Each of the obtained fungal antagonist and the pathogenic fungus was grown on potato dextrose agar (PDA) medium for 7 days at 25 ± 2 °C. Disk of individual antagonistic fungi and disk of each pathogenic fungi were placed on opposite sides of Petri plates containing the PDA medium (John et al. 2010). Inoculated plates were incubated for 7 days at 25 ± 2 °C. Five plates for each particular treatment were used as replicates. The plates were then examined and growth area of plates inoculated only with the pathogenic fungus was measured. The reduction percent in growth area of the pathogenic fungi was calculated using the formula as follows: reduction% = [(C − T)/C] × 100, where C is the average growth of pathogenic fungi in control treatment (cm2) and T is the average growth of pathogenic fungi in presence of antagonistic fungus (cm2).
Experimental design and treatments
Trichoderma harzianum, T. viride, T. virinis, and T. koningii were applied alone or in combination to study their effect on strawberry black root rot disease under field conditions. Actamyl 3 g/L (fungicide) and an untreated control were considered for comparison with the biocontrol efficacy as well as strawberry yield and enzyme activities. Experiments were carried out at fields previously known to be highly infested with strawberry black root fungi at El-Qalioubia governorate, Egypt. So, naturally infested plots (4 × 8 m), each comprised of 8 rows (32 holes/row and one seedling was sown in each hole), in a randomized complete block design with three replicates (plots) for each treatment were established. Strawberry seedlings were planted in loamy clay, well-drained soil to a depth of 10 cm. Irrigation and nutrition and other agricultural practices were carried out as recommended (El-Shemy et al. 2013). Before planting, strawberry seedlings were dipped in spore suspension (106 cfu/ml) of each Trichoderma species for 5 min as single and combined treatments. The rhizosphere of each plant also received 80 ml of spore suspension (106 cfu/ml) for each species alone or in combination every 15 days, i.e., six times for a total of 3 months during which black root rot disease of strawberry usually grows and spreads rapidly under natural conditions.
Disease assessment
The percentages of disease incidence were calculated 100 days after transplanting as follows:
Disease severity (DS) was recorded at the end of experiment according to original scale developed to evaluate a particular disease (Morocko 2006) as follows: 0 = plant well developed, no disease symptoms; 1 = no visible symptoms on above ground parts, 25% of roots discolored; 2 = plant slightly stunted, black necrosis on petiole bases, 26–50% of roots discolored; 3 = plant stunted, black necrosis on petiole bases, yellowing and death of outer leaves, 51–75% of roots discolored; 4 = plant severely stunted, outer leaves collapsed, younger leaves bluish green and wilting, > 75% of roots discolored; and 5 = plant dead.
\( \mathrm{Disease}\ \mathrm{severity}\%=\frac{\Sigma\ \left(\mathrm{Disease}\ \mathrm{grade}\times \mathrm{number}\ \mathrm{of}\ \mathrm{plants}\ \mathrm{in}\ \mathrm{each}\ \mathrm{grade}\right)}{\mathrm{Total}\ \mathrm{number}\ \mathrm{of}\ \mathrm{plants}\times \mathrm{highest}\ \mathrm{disease}\ \mathrm{grade}}\times 100 \)
Effects on plant growth and yield
Fresh and dray weight/plant were measured after 90 days of planting. Accumulated strawberry yield (tons/feddan) for each treatment was determined.
Determination of enzyme activities
Extraction of enzymes
Plant leaves (g) were homogenized with 0.1 M sodium phosphate buffer (pH 7.1) at the rate of 1/3 w/v (Goldschmidt et al. 1968). The homogenate was centrifuged at 3000 rpm for 15 min. The supernatant was used to determine activities of the following enzymes.
Peroxidase assay
Peroxidase activity was measured by incubating 0.1 ml of enzyme extract with 4 ml of guaicol solution for 1 min at 25 °C and absorbance at 470 nm. The guaicol solution consisted of 3 ml of 0.05 M potassium phosphate, pH 7, 0.5 ml of 2% guaicol, and 0.5 ml of 0.3% H2O2 (Abeles et al. 1971). Peroxidase activity was expressed as the increase in absorbance at 470 nm/gram fresh weight/1 min using spectrophotometer (Spectronic 20-D).
Chitinase assay
The substrate colloidal chitin was prepared from chitin powder according to the method described by Ried and Ogryd-Ziak (1981). Determination of chitinase activity was carried out according to the method of Monreal and Reese (1969). One milliliter of 1% colloidal chitin in 0.05 M citrate phosphate buffer (pH 6.6) in test tubes and 1 ml of enzyme extract were added and mixed by shaking. Tubes were kept in a water bath at 37 °C for 60 min, then cooled and centrifuged before assaying. Reducing sugar was determined in 1 ml of the supernatant by dinitrosalicylic acid. The reaction was stopped by heating the tubes for 5 min at 100 °C. The tubes were cooled and 3 ml of distilled water was added before assay. Optical density was determined at 540 nm using spectrophotometer (Spectronic 20-D). Chitinase activity was expressed as mM N-acetylglucoseamine equivalent released/gram fresh weight tissue/60 min.
Statistical analysis
Tukey test for multiple comparisons among means was utilized (Neler et al. 1985).
Results
Laboratory test
The results in Table 1 indicated that all tested species of Trichoderma significantly reduced growth area of the three pathogenic fungi. The highest reduction in growth area was obtained with T. harzianum, T. viride, and T. koningii which reduced the growth area by more than 90.6% for the tested fungi. Meanwhile, T. virinis was less effective.
Field experiment
Effect on strawberry disease incidence and severity
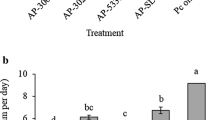
The results in Table 2 revealed that the four species of Trichoderma significantly reduced disease incidence and severity. The highest reduction was obtained with their combined mixture which reduced the disease incidence and severity by 83.3 and 88.5% respectively. Individual treatment of T. harzianum, T. viride, and T. koningii resulted in reducing the disease incidence and severity by more than 70.8 and 74% respectively. Meanwhile, T. virinis was less effective.
Effect on some vegetative characters
The results in Table 3 showed that all tested species of Trichoderma significantly increased fresh and dry weight of strawberry plants. The highest increase was obtained with the mixture (T. harzianum + T. viride + T. virinis + T. koningii) which increased the fresh and dry weight by 83.3 and 176.9%, respectively. Individual treatment of T. harzianum, T. viride, and T. koningii increased fresh and weight by more than 105 and 68%, respectively. Meanwhile, T. virinis was less effective.
Effect on strawberry yield
The results in Table 4 indicate that all tested species of Trichoderma significantly increase strawberry yield. The highest increase was obtained with mixture of (T. harzianum + T. viride + T. virinis + T. koningii) which increased the yield by 117.1%. Individual treatment of T. harzianum, T. viride, and T. koningii resulted in increasing yield by 71.1, 57.1, and 64.3% respectively. Meanwhile, T. virinis was less effective.
Effect on enzyme activities
The results in Table 5 revealed that all tested Trichoderma species significantly increased activities of strawberry enzymes. The highest increase was obtained with their mixture which enhanced the peroxidase and chitinase activity by 150 and 160.9%, respectively. Individual treatment of T. harzianum, T. viride, and T. koningii increased peroxidase and chitinase activity by more than 100 and 117.4%, respectively. Meanwhile, T. virinis was less effective.
Discussion
Black root rot is a complex disease caused several fungi (Fang et al. 2012; Hutton et al. 2013; Juber et al. 2014). This complex disease is characterized by feeder rootlet killing, deterioration and blackening of the main root system, and a decline in vigor and productivity of the plant stand causing damage to the host and considerable reduction in the yield (Abdet-Sattar et al. 2008; Ceja-Torres et al. 2014). Using T. harzianum and T. viride as seedling dressing for strawberry suppression of root rot and improving yield components has long been tried (Sullivan 2004). Results in the present study indicated that all tested species of Trichoderma significantly reduced growth area of the pathogenic tested fungi. A considerable reduction in growth area was obtained with T. harzianum, T. viride, and T. koningii. Field experiment results revealed that all tested species of Trichoderma significantly reduced disease incidence and severity. The highest reduction was obtained with their mixture. In this respect, Trichoderma species have been known to evolve numerous mechanisms for enhancing plant and root growth (Harman and Bjorkman 1998). Thus, the colonization of the root system by rhizosphere competent strains of Trichoderma may result in increasing development of roots and/or aerial systems and crop yields. Other activities like the induction of plant systemic resistance and antagonistic effects on plant pathogenic nematodes have also been proposed (Sharon et al. 2001; Roatti et al. 2013; Harel et al. 2014). It is probable that during the plant-Trichoderma interactions, the fungus participates actively in protecting and improving its ecological niche. Such dual roles make Trichoderma strains appealing alternatives to soil fumigation technologies such as methyl bromide. Strains of Trichoderma may also be aggressive bio-degraders and act as competitors to fungal pathogens in their saprophytic phases, especially when nutrients are a limiting factor (Simon and Sivasithamparam 1989; Mansoori et al. 2013; Angelopoulou et al. 2014). Such strains have been reported as promoting activities of nonpathogenic bacteria and mycorrhizal fungi (Calvet et al. 1993). The ability of Trichoderma strains to synthesize substances inducing systemic acquired resistance (SAR)-like responses in plants was shown (Enkerli et al. 1999; Harel et al. 2014). The combined systemic bio-fungicides and plant growth promoters have great market potential if the molecular basis of the activities can further be identified. The strong biodegradation and substrate colonization performances of Trichoderma strains is the result of an amazing metabolic versatility and a high secretory potential which leads to the production of a complex set of hydrolytic enzymes. Similarly, the myco-parasitic process is based on the secretion of a rich cocktail of cell wall degrading enzymes able to hydrolize the cell wall of various fungi, i.e., chitinases, β-1,3-glucanases, and proteases (Benítez et al. 1998). Some lytic enzymes can be involved in both antagonistic and saprophytic processes providing an evolutionary advantage to strains with both biodegrading and antagonistic potential, for the efficient colonization of different ecological niches in soil (Tziros et al. 2007). In the present study, all tested species of Trichoderma significantly increased strawberry enzymes. The highest increase was obtained with their mixture which may be due to complementary effects.
These results are in harmony with those obtained by others (Cherif et al. 2007; Prlak and Kose 2009; Saksirirat et al. 2009). The increase of yield also might be due to the protected healthy root system that absorb and supply adequate amount of raw nutrient and/or the syntheses of these raw nutrient materials effectively in presence of high amount of chlorophyll and protein, leading to more fruit yield (Shoresh and Harman 2008 and Akladious and Abbas 2012). Also, our results agreed with others (Cherif et al. 2007; Latha et al. 2009; Prlak and Kose 2009; Saksirirat et al. 2009) concerning the enhanced activities of chitinase and peroxidase in treated strawberry plants compared to untreated ones. Likewise, some other results recorded that the efficacy of Trichoderma species on soil borne fungal diseases is even higher than fungicides and maintain longer (e.g., Ha 2010; Heydari and Pessarakli 2010)
Conclusion
Despite the continuous progress in cultivation and production of strawberry in Egypt in recent years due to its economic and social benefits, great yield losses by many pests and diseases are frequently associated with its plants. Black root rot disease of strawberry, caused by the fungi Fusarium solani, Rhizoctonia solani, and Pythium sp., ranks high among current serious diseases of strawberry. Chemical fungicides may be used to control the disease, but the current search aspires to use safe control measures with a clear aim at the avoidance of human health hazards and environmental pollutions caused by chemicals. So, biocontrol agents were tried herein as safe alternatives. Trichoderma harzianum, T. viride, and T. koningii considerably reduced the growth area for all such pathogenic fungi. These four species significantly reduced black root rot disease incidence and severity under field conditions. The highest reduction was attained by mixture of the four species. Individual treatment of T. harzianum, T. viride, and T. koningii reduced both disease incidence and severity more than 70.8 and 74%, respectively. Moreover, this mixture considerably increased the strawberry fresh and dry weight as well as the yield. Also, Trichoderma species tested herein significantly increased two plant defense-related enzymes against pathogens in strawberry plants, i.e., peroxidase and chitinase activity. Thus, integrated pest management programs in ways that make these biocontrol agents complementary or superior to chemical fungicides should further be examined.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Abbreviations
- cfu:
-
Colony-forming units
- PDA:
-
Potato dextrose agar
- PDB:
-
Potato dextrose broth
References
Abd-Elgawad MMM (2017) Status of entomopathogenic nematodes in integrated pest management strategies in Egypt. In: Abd-Elgawad MMM, Askary TH, Coupland J (eds) Biocontrol Agents: Entomopathogenic and Slug Parasitic Nematodes. Wallingford, CAB International, UK, pp 473–501
Abd-Elgawad MMM (2019) Plant-parasitic nematodes of strawberry in Egypt: A Review. Bull NRC 43:7 https://doi.org/10.1186/s42269-019-0049-2
Abd-Elgawad MMM, Askary TH (2018) Fungal and bacterial nematicides in integrated nematode management strategies. Egypt J Biol Pest Cont 28:74 https://doi.org/10.1186/s41938-018-0080-x
Abd-Elgawad MMM, Vagelas IK (2015) Nematophagous bacteria: field application and commercialization. In: Askary TH, Martinelli PRP (eds) Biocontrol Agents of Phytonematodes. CAB International, Wallingford, pp 276–309
Abd-El-Kareem F, Elshahawy IE, Abd-Elgawad MMM (2019a) Management of strawberry leaf blight disease caused by Phomopsis obscurans using silicate salts under field conditions. Bull NRC 43:1 https://doi.org/10.1186/s42269-018-0041-2
Abd-El-Kareem F, Elshahawy IE, Abd-Elgawad MMM (2019b) Effectiveness of silicon and silicate salts for controlling black root rot and induced pathogenesis-related protein of strawberry plants. Bull NRC 43:91 https://doi.org/10.1186/s42269-019-0139-1
Abdet-Sattar MA, El-Marzoky HA, Mohamed AI (2008) Occurrence of soilborne diseases and root knot nematodes in strawberry plants grown on compacted rice straw bales compared with naturally infested soil. J Pl Prot Research 48(2):223–235
Abeles FB, Bosshart RP, Forrence LE, Habig WH (1971) Preparation and purification of glucanase and chitinase from bean leaves. Pl Physiol 47:129–134
Ahmed MFA, El-Fiki IAI (2017) Effect of biological control of root rot diseases of strawberry using Trichoderma spp. Middle East J Appl Sci 7:482–492
Akladious SA, Abbas SM (2012) Application of Trichoderma harziunum T22 as a biofertilizer supporting maize growth. Afr J. Biotechnol 11:8672–8683
Angelopoulou DJ, Naska EJ, Paplomatas J, Tjamos SA (2014) Biological control agents (BCAs) of Verticillium wilt: influence of application rates and delivery method on plant protection, triggering of host defence mechanisms and rhizosphere populations of BCAs. Pl Pathol 63(5):1062–1069. https://doi.org/10.1111/ppa.12198
Benitez T, Limon C, Delgado J, Rey M (1998) Glucanolytic and other enzymes and their genes. In: Kubicek CP, Harman GE (eds) Trichoderma and Gliocladium, vol 2. Taylor and Francis, London, pp 101–127
Calvet C, Ppera J, Barea M (1993) Growth response of marigold (Tagetes erecta L.) to inoculation with Glomus mosseae, Trichoderma aureoviride and Pythium ultimum in a peat-perlite mixture. Pl Soil 148:1–6
Ceja-Torres LF, Mora-Aguilera G, Mora-Aguilera A (2014) Agronomical management influence on the spatiotemporal progress of strawberry dry wilt in Michoacán, Mexico. Afr J Agric Res 9(4):513–520
Cherif M, Arafaoui A, Rhaiem A (2007) Phenolic compounds and their role in bio-control and resistance of chickpea to fungal pathogenic Attacks. Tunisian J Pl Prot 2:7–21
El-Mougy NS, Abdel-Kader MM (2014) Integration of urea fertilizer and Trichoderma harzianum for controlling Rhizoctonia root rot disease of lupine under field conditions. Int J Eng Innov Tech 4(2):213–217
Elshahawy IE, Saied-Nehal M, Abd-El-Kareem F, Morsy AA (2017) Biocontrol of onion white rot by application of Trichoderma species formulated on wheat bran powder. Archives Phytopath Pl Prot 50(3-4):150–166
El-Shemy AA, Khafagy YS, Al-Genteery AMM (2013) Cultivation and production of strawberry. Techn issue no. 9/2013, General Directorate of Agricultural Culture, Egyptian Ministry of Agriculture, Giza, p 135 (in Arabic)
Enkerli J, Felix G, Boller T (1999) Elicitor activity of fungal xylanase does not depend on enzymatic activity. Pl Physiol 121:391–398
Fang XD, Kuo J, You MP, Finnegan PM, Barbetti MJ (2012) Comparative root colonization of strawberry cultivars Camarosa and Festival by Fusarium oxysporum f. sp. fragariae. Pl Soil 358:75–89
Goldschmidt EE, Goren R, Monselise SP (1968) The indol acetic acid oxidase system of citrus roots. Planta 72:213–222
Ha TN (2010) Using Trichoderma species for biological control of plant pathogens in Vietnam. J ISSAAS 16(1):17–21
Harel YL, Mehari ZH, David D, Elad Y (2014) Systemic resistance to gray mold induced in tomato by benzothiadiazole and Trichoderma harzianum T39. Phytopathology 104:150–157
Harman GE, Bjorkman T (1998) Potential and existing uses of Trichoderma and Gliocladium for plant disease control and plant growth enhancement. In: Kubicek CP, Harman GE (eds) Trichoderma and Gliocladium, vol 2. Taylor and Francis, London, pp 229–265
Harman GE, Howell CR, Viterbo A, Chet I, Lorito M (2004) Trichoderma species opportunistic a virulent plant symbionts. Nat. Rev. Microbiol 2:43–56
Hernandez SM, Castillo FD, Morales G, Saldivar RH, Aguilar CN (2011) Biocontrol of soil fungi in tomato with microencapsulates containing Bacillus subtilis. American J Agric. Biol. Sci 6:189–195
Heydari A, Pessarakli M (2010) A review of biological control of fungal plant pathogens using microbial antagonists. J Biol Sci 10(4):273–290
Hutton DG, Gomez AO, Mattner SW (2013) Macrophomina phaseolina and its association with strawberry crown rot in Australia. Int J Fruit Sci 13(1-2):149–155
John PR, Tyagi RD, Prevost D, Pouleur S, Surampalli RY (2010) Mycoparasitic Trichoderma viride as a biocontrol agent against Fusarium oxysporum f. sp. adzuki and Pythium arrhenomanes and as a growth promoter of soybean. Crop Prot 29:1452–1459
Juber KS, Al-Juboory HH, Al-Juboory SB (2014) Fusarium wilt disease of strawberry caused by Fusarium oxysporum f. sp. Fragariae in Iraq and its control. J Exper Biol Agric Sci 2(4):419–427
Karlidag H, Esitken A, Yildirim E, Donmez M, Turan F (2012) Effects of plant growth promoting bacteria on yield, growth, leaf water content, membrane permeability, and ionic composition of strawberry under saline conditions. J Pl Nutrit 34:34–45
Latha PA, Ragupathi NT, Samiyappan NT (2009) Antimicrobial activity of plant extracts and induction of systemic resistance in tomato plants by mixtures of PGPR strains and Zimmu leaf extract against Alternaria solani. Biol Cont 50:85–93
Mansoori M, Nader A, Rezaee S, Naraghi L (2013) Evaluation of Pseudomonas and Bacillus bacterial antagonists for biological control of cotton Verticillium wilt Disease. J Pl Prot Res 53:154–157
Monreal J, Reese ET (1969) The chitinase of Serratia marcescens. Canad J Microbiol 15:689–696
Morocko I (2006) Characterization of the strawberry pathogen Gnomonia fragariae, and biocontrol possibilities. Ph. D. dissertation, Acta Universitatis agriculturae Sueciae, Uppsala, Sweden.
Neler J, Wassermann W, Kutner MH (1985) In: Richard D (ed) Applied linear statistical models. Regression, analysis of variance and experimental design, 2nd edn. Irwin Inc, Homewood
Prlak L, Kose M (2009) Effects of plant growth promoting rhizobacteria on yield and some fruit properties of strawberry. J Pl Nutrit 32:1173–1184
Ried JD, Ogryd-Ziak DM (1981) Chitinase over producing mutant of Servatia marcescens. Appl Environ Microbiol 41:664–669
Roatti B, Perazzolli M, Gessler C, Pertot I (2013) Abiotic stresses affect Trichoderma harzianum T39-induced resistance to downy mildew in grapevine. Phytopathology 103:1227–1234
Saksirirat W, Chareerak P, Bunyatrachata W (2009) Induced systemic resistance of biocontrol fungus, Trichoderma spp. against bacterial and gray leaf spot in tomatoes. Asian J food and Agro-Indust, Special issue, pp 99-104.
Sharon E, Bar-Eayal M, Spiegel Y (2001) Biocontrol of the root-knot nematode Meloidogyne javanica by Trichoderma harzianum. Phytopathology 91:687–693
Shehata IE, Hammam MMA, El-Borai FE, Duncan LW, Abd-Elgawad MMM (2019) Comparison of virulence, reproductive potential, and persistence among local Heterorhabditis indica populations for the control of Temnorhynchus baal (Reiche & Saulcy) (Coleoptera: Scarabaeidae) in Egypt. Egypt J Biol Pest Cont 29:32 https://doi.org/10.1186/s41938-019-0137-5
Shoresh M, Harman GE (2008) The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root. Pl Physiol 147(4):2147–2163
Simon A, Sivasithamparam K (1989) Pathogen suppression: a case study in biological suppression of Gaeumannomyces graminis var. tritici in soil. Soil Biol Biochem 21:331–337
Sullivan P (2004) Sustainable management of soil-borne plant diseases soil systems guide, National Sustainable Agriculture Information Service NCAT Agriculture Specialist, US Department of Agriculture, p 173
Tziros G, Lagopodi A, Tzavella-Klonari K (2007) Reduction of Fusarium wilt in watermelon by Pseudomonas chlororaphis PCL1391 and P.fluorescens WCS365. Phytopathologia Mediterranea 46:320–323
Ziedan EHE, Elewa IS, Mostafa MH, Sahab AF (2011) Application of mycorrhizae for controlling root diseases of sesame 51(4):355-361
Ziedan EHE, Farragb ESH, Sahab AF (2015) Effect of Trichoderma harzianum against Thielaviopsis paradoxa and their pathological potential on date palm seedlings. Int J Agric Tech 11(4):913–923
Acknowledgements
This study was supported in part by the US-Egypt Project cycle 17 (no. 172) entitled “Preparing and evaluating IPM tactics for increasing strawberry and citrus production.” This article is derived from the Subject Data funded in part by NAS and USAID, and any opinions, findings, conclusions, or recommendations expressed in it are those of the authors alone and do not necessarily reflect the views of USAID or NAS. Facilities offered by The National Research Centre are appreciated.
Funding
Financial support made by US-Egypt Project fund for Project cycle 17 (no. 172) and National Research Centre, Egypt, is gratefully acknowledged.
Author information
Authors and Affiliations
Contributions
All authors participated in the development and implementation of the research plan and subsequently written it. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Abd-El-Kareem, F., Elshahawy, I.E. & Abd-Elgawad, M.M.M. Local Trichoderma strains as a control strategy of complex black root rot disease of strawberry in Egypt. Bull Natl Res Cent 43, 160 (2019). https://doi.org/10.1186/s42269-019-0206-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0206-7