Abstract
Background
The use of nanotechnology can ensure food security via improving crop production. Nanoparticles have the ability to enhance growth and yield of different plants such as fenugreek (Trigonella foenum-graecum) (Fabaceae). The present work aims to study the role of silver nanoparticles (AgNPs) on growth, some biochemical aspects, and the yield both quantitatively and qualitatively of fenugreek plant. AgNPs were synthesized by chemical reduction of silver nitrate with trisodium citrate.
Results
Foliar application of AgNPs with different concentrations (20, 40, and 60 mg/l) improved the growth parameters of fenugreek plant (e.g., shoot length, number of leaves/plant, and shoot dry weight) and increased some biochemical aspects such as photosynthetic pigment (chlorophyll a, chlorophyll b, and carotenoids) and indole acetic acid (IAA) contents thus enhanced the yield quantity (number of pods/plant, number of seeds/pod, weight of seeds/plant, and seed index) and quality (carbohydrate%, protein%, phenolics, flavonoids, and tannins contents) of the yielded seeds as well as increasing antioxidant activity of the yielded seeds.
Conclusion
The most effective treatment was 40 mg/l as it caused the highest increases in the studied parameters.
Similar content being viewed by others
Introduction
Fenugreek (Trigonella foenum-graecum) is an annual herb that belongs to the family Leguminosae widely grown in Egypt and Middle Eastern countries. Due to its strong flavor and aroma, fenugreek is one of such plants whose leaves and seeds are widely consumed as a spice in food preparations and as an ingredient in traditional medicine (Abd Elhamid et al. 2016). It is a rich source of calcium, iron, â-carotene, and other vitamins (Sharma et al. 1996). Both leaves and seeds should be included in the normal diet of the family, especially diet of growing children, pregnant women, puberty-reaching girls, and elder members of the family, because they have hematinic (i.e., blood formation) value (Ody 1993). Fenugreek seeds contain lysine- and l-tryptophan-rich proteins, mucilaginous fiber, and other rare chemical constituents such as saponins, coumarin, fenugreekine, nicotinic acid, sapogenins, phytic acid, scopoletin, and trigonelline which are thought to account for many of fenugreek presumed therapeutic effects. Fenugreek might inhibit cholesterol absorption to help to lower sugar levels (Bukhari et al. 2008). In addition, all extracts of the fenugreek seeds (methanol, ethanol, dichloromethane, acetone, hexane, and ethyl acetate) exhibit antioxidant activity because of the phenolic acids and flavonoids. The phenolic compounds ranged from 1.35 to 6.85 mg/g, and the total flavonoids are in the range from 208 to 653 μg/g according to the extract type (Bukhari et al. 2008). Furthermore, intercropping fenugreek with faba bean can reduce Orobanche crenata infection (Fernández-Aparicio et al. 2006).
Higher plants, as sessile organisms, have a remarkable ability to develop mechanism to perform better under suitable and unsuitable conditions. Nowadays, scientists/researchers want to develop new techniques that could be suitable for plants to boost their native functions. Nanoparticles (NPs) are microscopic particles with at least one dimension less than < 1000 nm. For this, these particles are very attractive materials to handle in biological system. NPs are found to be very suitable in sensing and detection of biological structures and systems (Singh et al. 2008). NPs show a promise in different fields of agricultural biotechnology (Majumder et al. 2007). NPs have unique physicochemical properties and the potential to boost the plant metabolism (Giraldo et al. 2014). Using different fertilizers are very important for plant growth and development, but most of the used fertilizers are rendered unavailable to plants due to many factors, such as leaching, degradation by photolysis, hydrolysis, and decomposition. Therefore, it is necessary to minimize nutrient losses in fertilization and to increase the crop yield through the exploitation of new applications with the help of nanotechnology and nanomaterials. Nano-fertilzers or nano-encapsulated nutrients might have properties that are effective to crops, release the nutrients on demand, control the release of chemical fertilizers that regulate plant growth, and enhance target activity (DeRosa et al. 2010 and Nair et al. 2010). Agricultural application of NPs is currently an interesting area of interest for minimizing the use of chemical fertilizers and improves growth and yield of crops (Majumder et al. 2007; Lee et al. 2008; Siddiqui and Al-Whaibi 2014). The introduction of nanoparticles into plants might have a significant impact, and thus, they can be used for agricultural applications for better growth and yield (Josko and Oleszczuk 2013). However, a thorough understanding of the role of nano-sized engineered materials in plant physiology at the molecular level is still lacking (Khodakovskaya et al. 2011) whereas the mode of action of nanoparticles on plant growth and development is still too scarce. Plants under certain conditions were reported to be capable of producing natural mineralized nanomaterials necessary to their growth (Wang et al. 2001). Seed germination provides a suitable foundation for plant growth, development, and yield (Siddiqui and Al-Whaibi 2014).
AgNPs are currently the most produced engineered nanomaterials found in a wide range of commercial products (Davies 2009). AgNPs have been implicated in agriculture for improving crops. There are several reports indicating that appropriate concentrations of AgNPs play an important role in enhancing seed germination (Barrena et al. 2009; Shelar and Chavan 2015) and plant growth (Sharma et al. 2012; Kaveh et al. 2013; Vannini et al. 2013), improving photosynthetic quantum efficiency and chlorophyll content (Sharma et al. 2012; Hatami and Ghorbanpour 2013), and increasing water and fertilizer use efficiency (Lu et al. 2002).
Therefore, the aim of this work is to study the effect of using AgNPs in enhancing growth, some biochemical aspects, yield quantity, and some nutritional values of fenugreek plant.
Materials and methods
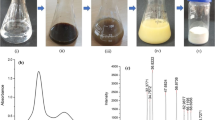
Silver nanoparticles (AgNPs) were synthesized by the reduction of silver nitrate (AgNO3) with tri-sodium citrate (Na3C6H5O7·2H2O) according to the methods described (Kulkarni 2007) with little modification. Silver nitrate (510 mg) was dissolved in 500 ml distilled water and heated for 15 min at 75–80 °C with continuous stirring at 7000 rpm on a magnetic stirrer. Then, 500 ml solution containing 300 mg of trisodium citrate was added slowly. The solution was kept at 75–80 °C with continuous stirring for about 1 h. When the solution turned golden yellow (indication of silver nanoparticles), the reaction was stopped by adding ascorbic acid at 1.0 mg/l and the AgNPs were stabilized. Aggregation of AgNPs was not observed. Furthermore, reaction conditions and concentration of the reactants were adjusted in such way to ensure that no silver ions were left in the solution. The solution so prepared was used in experiments.
Seeds of fenugreek cv. Giza 30 were obtained from the Agriculture Research Centre (ARC), Ministry of Agriculture and Land Reclamation, Egypt. The seeds were grown in pots (diameter 30 cm in diameter) at two successive seasons (2014/2015 and 2015/2016), filled with clay and sand with the ratio of 2:1. AgNP concentrations (0, 20, 40, and 60 mg/l) were sprayed after 30 and 37 days of sowing. Superphosphate (5 g/pot), potassium sulfate (2.5 g/pot), and urea (6 g/pot) were used for fertilization.
The pot experiment was conducted in the greenhouse of the Botany Department, NRC. The experimental design used was complete randomized blocks. Samples were taken after 60 days after sowing (DAS) to analyze the crop performance in terms of growth parameters, indole acetic acid (IAA), photosynthetic pigment (chlorophyll a, chlorophyll b, and carotenoids). Each treatment was replicated four times, and each replicate had three plants. Three healthy plants were left in each pot to determine the number of pods/plant, number of seeds/pod, weight of seeds/plant, and seed index (SI). Air-dried seeds were powdered and kept in desiccators for chemical analysis.
Photosynthetic pigments (chlorophyll a, chlorophyll b, and carotenoids) of fresh leaves were determined according to Moran (1982). Indole acetic acid content was extracted and analyzed using the method of Larsen et al. (1962). The seed powder was used to determine proteins, carbohydrates, phenolic, flavonoids, and tannins contents. Protein content was determined by micro-Kjeldahl method according to Miller and Houghton (1945). Total carbohydrates were determined colorimetrically according to the method of Dubois et al. (1956). Total phenol content was measured as described by Danil and George (1972). Total flavonoid contents were measured by the aluminum chloride colorimetric assay as described by Ordoñez et al. (2006). Tannins were determined using the modified vanillin hydrochloric acid (MV-HCl) according to Brand-Williams et al. (1995) using the 1.1-diphenyl-2-picrylhydrazil (DPPH) reagent.
Statistical analysis
The results were statistically analyzed using the MSTAT-C (1988) software. The mean comparisons among treatments were determined by Duncan’s multiple range test at 5 P ≤ 0.05 (Gomez and Gomez 1984).
Results
Plant growth
The data presented in Table 1 shows the effect of different concentrations of AgNps on shoot length, number of leaves/plant, and shoot dry wt. of fenugreek plants. Foliar application of fenugreek plants with different AgNP concentrations increased all these growth parameters as compared with the untreated plants. Data clearly revealed the gradual increase of shoot length, number of leaves/plant, and shoot dry wt. in response to increased AgNP concentrations from 0 to 20 and 40 mg/l. At 60 mg/l, the response decreased but still higher than the control. The highest response in all the growth criteria was obtained by using 40 mg/l AgNPs.
Photosynthetic pigments
Data in Table 2 show the response of photosynthetic pigments of fenugreek leaves sprayed with different concentrations of AgNPs. The results revealed the significant increases in all photosynthetic pigment contents (chlorophyll a, chlorophyll b, carotenoids, and total pigments) in response to treatment with different concentrations of AgNPs. Increased AgNP concentrations resulted in a significant increase in photosynthetic pigments gradually up to 40 mg/l. Treatment with 60 mg/l decreased photosynthetic pigment contents, when compared with the other two lower concentrations, but still higher than the untreated control. The most effective treatment was 40 mg/l AgNPs as it gave the highest increases in all photosynthetic pigments.
Endogenous IAA
Table 2 shows the variation in growth promoter (IAA) in response to spraying with different concentrations of AgNPs. Spraying fenugreek plants with different concentrations of AgNPs significantly increased IAA. The most effective treatment was 40 mg/l of AgNps as it resulted in the highest content of IAA compared with the corresponding control plants.
Yield and yield components
Results in Table 3 illustrate the behavior of fenugreek plant under the effect of foliar application of different concentrations of AgNPs on yield and yield components. Treatments showed that different concentrations of AgNPs increased yield components of fenugreek as compared with the control plant. The most effective treatment was also 40 mg/l of AgNPs.
Biochemical constituents of the yielded fenugreek seeds
Table 4 demonstrates that foliar AgNP treatment of fenugreek plant led to marked increases in total carbohydrates% and protein% when compared with control plants; these increases reached maximum levels again at 40 mg/l AgNPs. Table 4 also shows the variation in antioxidant substances of the yielded seeds in response to spraying with different concentrations of AgNPs. Treatments caused significant increases in both total phenolic and flavonoids as well as tannin contents as compared with those of the control. Regarding antioxidant activity, foliar treatment with AgNPs with different concentrations caused significant increases in antioxidant activity of the yielded seeds of fenugreek plant.
Discussion
Data presented in Table 1 shows that foliar application of fenugreek plants with different concentrations of AgNPs increased all growth criteria (shoot length, number of leaves/plant, and shoot dry wt.) when compared with the untreated plants. More or less similar data were observed by Salama (2012b) who noticed that low concentrations of AgNPs had a stimulating effect on the growth of the common bean and corn plants. Latif et al. (2017) showed that AgNP foliar treatment with different concentrations increased growth parameters of the wheat plant. The induced growth increases caused by different AgNP concentrations especially at 40 mg/l might be due to the role of AgNPs in blocking ethylene signaling in fenugreek plant (Rezvani et al. 2012). The impact of AgNPs on the morphology and physiology of plants depends on the size and shape of NPs. Syu et al. (2014) studied the effect of three different morphologies of AgNPs on physiological and molecular response of Arabidopsis and suggested that decahedral AgNPs showed the highest degree of root growth promotion (RGP); however, the spherical AgNPs had no effect on RGP and triggered the highest levels of anthocyanin accumulation in Arabidopsis seedlings. Moreover, Nghia et al. (2017) confirmed this positive effect of AgNPs.
The results revealed the significant increases in all photosynthetic pigment contents (chlorophyll a, chlorophyll b, carotenoids, and total pigments) in response to treatment with different concentrations of AgNPs. This is in line with the results obtained by Farghaly and Nafady (2015) and Latif et al. (2017) who reported that AgNPs significantly promote photosynthesis and it is closely related to the change of nitrogen metabolism. Also, Racuciu and Creange (2007) reported that chlorophyll content of maize plants was increased with low concentration (10–50 μl/l) of AgNP treatment while it was found to be inhibited at higher concentrations of NPs. According to Govorov and Carmeli (2007), metal nanoparticles can induce the efficiency of chemical energy production in photosynthetic systems. However, higher content of photosynthetic pigments, i.e., chlorophyll a, chlorophyll b, and carotenoids, would increase the rate of photosynthesis, due to which there was more production of photosynthesis process, which in turn increased the weight and growth of plant as it was observed in our study.
Spraying fenugreek plant with AgNPs showed that different concentrations of AgNPs increased the yield components compared with the control plant. The most effective treatment was 40 mg/l of AgNPs. Increase in yield by the application of nanoparticles has been postulated earlier. Silver is an excellent growth simulator (Sharon et al. 2010). Similar results of improving the role of AgNP treatments were obtained on mung bean by Najafi and Jamei (2014) and Razzaq et al. (2016) on wheat plant. These increases in yield and yield components might be attributed to the increases in growth parameters, photosynthetic pigments, and IAA of treated fenugreek plants.
Recently, Krishnaraj et al. (2012) found that biosynthesized AgNPs showed a significant effect on seed germination and induced the synthesis of protein and carbohydrate and decreased the total phenol contents of Bacopa monnieri. AgNPs increased plants’ growth profile and biochemical attributes (chlorophyll, carbohydrate and protein contents, antioxidant enzymes) of Brassica juncea, common bean, and corn (Salama 2012a; Sharma et al. 2012). The impact of AgNPs on the morphology and physiology of plants depends on the size and shape of NPs.
Conclusion
The present work demonstrated the effect of silver nanoparticles on fenugreek plant. Different concentrations increased plant growth, photosynthetic pigments, IAA contents, and yield quantity and quality. Among various concentrations used in the study, 40 mg/l AgNPs was the most effective treatment for the improvement in growth, biochemical parameters studied, and yield of fenugreek.
References
Abd Elhamid EM, Sadak MS, Tawfik MM (2016) Physiological response of fenugreek plant to the application of proline under different water regimes. Res. J. of Pharmaceutical, Biol. and Chem. Sci. 7(3):580–594
Barrena R, Casals E, Colon J, Font X, Sanchez A, Puntes V (2009) Evaluation of the ecotoxicity of model nanoparticles. Chemosphere 75:850–857
Brand-Williams, W., M. E. Cuvelier, and C. Berset. (1995). Use of a free radical method to evaluate antioxidant activity. LWT-Food Science and Technology, 28(1): 25–30.
Bukhari SB, Bhanger MI, Memon S (2008) Antioxidative activity of extracts from fenugreek seeds (Trigonella foenum-graecum). Pak. J. Environ. Chem. 9:78–83
Danil AD, George CM (1972) Peach seed dormancy in relation to endogenous inhibitors and applied growth substances. J. Amer. Soc. Hort. Sci. 17:621–624
Davies JC (2009) Nanotechnology oversight: an agenda for the new administration. Project on emerging technologies. Woodrow Wilson International Center for Scholars, Washington, DC
DeRosa MC, Monreal C, Schnitzer M, Walsh R, Sultan Y (2010) Nanotechnology in fertilizers. Nat Nanotechnol 5:91. https://doi.org/10.1038/nnano.2010.2
Dubois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Colorimetric method for determination of sugars and related substances. Analytical Chem 28(3):351–356
Farghaly FA, Nafady NA (2015) Green synthesis of silver nanoparticles using leaf extract of Rosmarinus officinalis and its effect on Tomato and wheat plants. J Agric Sci 7(11). https://doi.org/10.5539/jas.v8n4p179
Fernández-Aparicio M, Andolfi A, Evidente A, Rubiales D (2006) Orobanche species specific responses to Trigonella foenum-graecum root exudates. In: Workshop Parasitic Plant Management in Sustainable Agriculture Final meeting of COST849. 23-24 November 2006, ITQB Oeiras-Lisbon, Portugal p.10
Giraldo JP, Landry MP, Faltermeier SM, McNicholas TP, Iverson NM, Boghossian AA, Reuel NF, Hilmer AJ, Sen F, Brew JA, Strano MS (2014) Plant nanobionics approach to augment photosynthesis and biochemical sensing. Nat Mater. 13(4):400–408. https://doi.org/10.1038/nmat3890
Gomez KA, Gomez AA (1984) Statistical procedures for agricultural research. John Wiley & Sons Inc., Singapore, 680.
Govorov AO, Carmeli I (2007) Hybrid structures composed of photosynthetic system and metal nanoparticles: plasmon enhancement effect. Nano Lett. 7(3):620–625
Hatami M, Ghorbanpour M (2013) Effect of nanosilver on physiological performance of pelargonium plants exposed to dark storage. J. Hort. Res. 21:15–20
Josko I, Oleszczuk P (2013) Influence of soil type and environmental conditions on ZnO, TiO2 and Ni nanoparticles phytotoxicity. Chemosphere 92:91–99
Kaveh R, Li YS, Ranjbar S, Tehrani R, Brueck CL, Van Aken B (2013) Changes in Arabidopsis thaliana gene expression in response to silver nanoparticles and silver ions. Environ. Sci. Technol. 47:10637–10644
Khodakovskaya MV, Silva K, Nedosekin DA, Dervishi E, Biris AS, Shashkov EV, Ekaterina IG, Zharov VP (2011) Complex genetic, photo thermal, and photo coustic analysis of nanoparticle-plant interactions. Proceeding of Natural Academy Science 108(3):1028–1033
Krishnaraj C, Jagan EG, Ramachandran R, Abirami SM, Mohan N, Kalaichelvan PT (2012) Effect of biologically synthesized silver nanoparticles on Bacopa monnieri (Linn.) Wettst. Plant growth metabolism. Process Biochem 47(4):51–658
Kulkarni SK (2007) Nanotechnology: principles and practices. Capital Pub. Co., India
Larsen PA, Harbo S, Klungron ATA (1962) On the biosynthesis of some indole compounds in Acetobacter xylinum. Physiol Plant 15:552–565
Latif HH, Ghareib M, Abu Tahon M (2017) Phytosynthesis of silver nanoparticles using leaf extracts from Ocimum basilicum and Mangifira indica and their effect on some biochemical attributes of Triticum aestivum Gesunde Pflanzen (2017), vol 69, pp 39–46
Lee WM, An YJ, Yoon H, Kweon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mungbean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environmental Toxicology and Chemistry 27(9):1915–1921
Lu C, Zhang C, Wen J, Wu G, Tao M (2002) Research of the effect of nanometer materials on germination and growth enhancement of Glycine max and its mechanism. Soybean Sci. 21:168–171
Majumder DD, Ulrichs C, Mewis I, Weishaupt B, Majumder D, Ghosh A, Thakur AR, Brahmachary RL, Banerjee R, Rahman A, Debnath N, Seth D, Das S, Roy I, Sagar P, Schulz C, Linh NQ, Goswami A (2007) Current status and future trends of nano-scale technology and its impact on modern computing biology medicine and agricultural biotechnology. In: Proceedings of the international conference on computing: theory and applications. IEEE Press, ICCTA, India, March, vol 5–7, pp 563–572
Miller L, Houghton JA (1945) The micro-Kjeldahl determination of the nitrogen content of amino acids and proteins. Biol. Chem. 159:373–383
Moran R (1982) Formula for determination of chlorophyllous pigments extracted with N.N. dimethylformamide. Plant Physiology 69:1371–1381
MSTAT-C (1988) MSTAT-C, a microcomputer program for the design, arrangement and analysis of agronomic research. Michigan State University, East Lansing
Nair R, Varghese SH, Nair BG, Maekawa T, Yoshida Y, Kumar DS (2010) Nanoparticulate material delivery to plants. Plant Sci 179:154–163
Najafi S, Jamei R (2014) Effect of silver nanoparticles and Pb(NO3)2 on the yield and chemical composition of mung bean (Vigna radiata). J. Stress Physiol. Biochem. 10:316–325
Nghia LT, Tung HT, Huy NP, Luan VQ, Nhut DT (2017) The effect of silver nanoparticles on growth of Chrysanthemum morifolium Ramat. cv. “JIMBA” in different cultural systems. Vietnam J. of Sci. and Tech 55(4):503–514. https://doi.org/10.15625/2525-2518/55/4/9322
Ody P (1993) New York: Dorling Kindersley, vol 47, p 164
Ordoñez AAL, Gomez JD, Vattuone MA, lsla MI (2006) Antioxidant activities of Sechium edule (Jacq.) Swartz extracts. Food Chemistry 97:452–458
Racuciu M, Creanga DE (2007) TMA-OH Coated magnetic nanoparticles internalized in vegetal tissue. Roman J Phys 52:395–402
Razzaq A, Ammara R, Jhanzab HM, Mahmood T, Hafeez A, Hussain S (2016) Anoval nanomaterial to enhance growth and yield of wheat. J of Nanoscience & Technology 2(1):55–58
Rezvani N, Sorooshzadeh A, Farhadi N (2012) Effect of nano-silver on growth of saffron in flooding stress. World Acad Sci Eng Technol 1:517–522
Salama HMH (2012a) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int. Res. J. Biotech. 3:190–197
Salama HMH (2012b) Effects of silver nanoparticles in some crop plants, common bean (Phaseolus vulgaris L.) and corn (Zea mays L.). Int Res. J Biotechnol 3:190–197
Sharma P, Bhatt D, Zaidi MG, Saradhi PP, Khanna PK, Arora S (2012) Silver nanoparticle-mediated enhancement in growth and antioxidant status of Brassica juncea. Appl. Biochem. Biotechnol. 167:2225–2233
Sharma RD, Sarkar A, Hazra DK (1996) Phytother. Res, vol 10, p 332
Sharon K, Choudhry A, Kumar R (2010) Nanotechnology in agricultural disease and food safety. J. Phytol. 2:83–92
Shelar GB, Chavan AM (2015) Myco-synthesis of silver nanoparticles from Trichoderma harzianum and its impact on germination status of oil seed. Biolife 3:109–113
Siddiqui MH, Al-Whaibi MH (2014) Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds. Mill.). Saudian J. Biol Sci 21:13–17
Singh M, Shinjini S, Prasad S, Gambhir IS (2008) Nanotechnology in medicine and antibacterial effect of silver nanoparticles. Digest J Nanomaterials and Biostructures 33:115–122
Syu YY, Hung JH, Chen JC, Chuang HW (2014) Impacts of size and shape of silver nanoparticles on Arabidopsis plant growth and gene expression. Plant Physiol Biochem 83:57–64
Vannini C, Domingo G, Onelli E, Prinsi B, Marsoni M, Espen L, Bracale M (2013) Morphological and proteomic responses of Eruca sativa exposed to silver nanoparticles or silver nitrate. PLoS One 8:e6875
Wang LJ, Guo ZM, Li TJ, Li M (2001) The nano structure SiO2 in the plants. Chin. Sci. Bull 46:625–631
Acknowledgements
Not applicable
Funding
Not applicable
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
The author shares every step of this work and wrote the manuscript. The author read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The author declares that there are no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Sadak, M.S. Impact of silver nanoparticles on plant growth, some biochemical aspects, and yield of fenugreek plant (Trigonella foenum-graecum). Bull Natl Res Cent 43, 38 (2019). https://doi.org/10.1186/s42269-019-0077-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42269-019-0077-y