Abstract
The current classification of renal cell carcinoma (RCC) was formulated at the meeting of the World Health Organization Renal Tumor Panel in 2015, with the results published in the fourth edition of the World Health Organization Classification of Tumours of the Urinary System and Male Genital Organs Bluebook in 2016. At that meeting a number of tumor types were designated as emerging or provisional entities as it was felt that they were insufficiently characterized to merit inclusion as a recognized type of RCC. One tumor type included in this designation was thyroid-like follicular RCC. Since the publication of the 2016 classification this tumor type has been further characterized and in addition to this, detailed studies on three other types of RCC (multifocal oncocytoma-like tumors associated with oncocytosis, eosinophilic solid and cystic RCC and biphasic squamoid alveolar RCC) have been published. It is now apparent that these four tumors are unique morphotypes and genotypes of RCC, and are likely to be included in the next edition of the World Health Organization classification of renal tumors. Multifocal oncocytoma-like tumors associated with oncocytosis is a benign process characterized by the presence of hundreds to thousands of oncocytic tumors in a single kidney. These tumors occur sporadically and are unrelated to the tumors of Birt-Hogg-Dubé syndrome. Eosinophilic solid and cystic RCC is characterized by a solid and cystic architecture with tumor cells consisting of bulky eosinophilic and granular cytoplasm with intracytoplasmic vacuolation. Thyroid-like follicular RCC occurs in younger patients with a female predominance. The tumor bears a striking resemblance to follicular carcinoma of the thyroid with follicles containing intraluminal proteineacous material resembling thyroglobulin. Immunostains for thyroid markers are negative. Finally, biphasic squamoid alveolar RCC consists of aggregates of large cells with pale eosinophilic cytoplasm usually arranged in a glomeruloid/alveolar pattern and surrounded by a border of basophilic cells with scanty cytoplasm. The genotype of the tumor, as well its recorded association with typical papillary RCC, has led to the suggestion that it is related to type 1 papillary RCC.
Similar content being viewed by others
Background
The classification of renal cell carcinoma (RCC) is a shifting landscape with novel forms of RCC being reported regularly. Prior to the 1980s our understanding that RCC is a large group of tumors with unique clinical, molecular and/or histological features was poorly developed and this is best illustrated in the 1981 Classification of the World Health Organization (WHO) which divided renal carcinoma into Renal Cell Carcinoma and Others (Delahunt & Eble, 2005).
The publication of the Mainz Classification in 1986, was a major step forward as this recognized that clear cell RCC, papillary RCC, chromophobe RCC and collecting duct carcinoma were all different tumor types (Thoenes et al., 1986). The classification also recognized that sarcomatoid RCC was a form of high grade tumor rather than a specific tumor type, which has led to the classification of sarcomatoid morphology as a feature of WHO/International Society of Urological pathology (ISUP) Grade 4 tumors under the WHO/ISUP grading system (Delahunt, 1999; Delahunt et al., 2013). A meeting to formulate an updated WHO classification for RCC was convened in 1989; however, there was no consensus as to the basis of the classification and the proposed classification, which failed to embrace many of the contemporary developments in our understanding of RCC, remained unpublished until 1998 (Mostofi et al., 1998). In the interim two consensus conferences were convened in Heidelberg (1996) and Rochester, Minnesota (1997) and this resulted in what has become known as the Heidelberg/Rochester Classification which incorporates the features of the Mainz recommendations (Kovacs et al., 1997; Stőrkel et al., 1997).
The third edition of the WHO Classification was formulated in 2002, being published in 2004 (Eble et al., 2004). This classification incorporated Xp11 translocation RCC, RCC-associated with neuroblastoma and mucinous tubular and spindle RCC, all of which having been recently characterized. The classification also included RCC Unclassified as a diagnostic category which was designed to incorporate tumors with features that were not typical of the recognized tumor types. Although this is not a specific diagnosis, the inclusion of the RCC Unclassified in the classification was an important development as it provided a designation for tumors that did not have the features of the recognized types of RCC. It is from this group of unclassified RCC that many of the more recently described types of RCC have been categorized (Delahunt & Eble, 2005).
The classification of RCC was updated at a consensus conference convened under the auspices of the ISUP in 2012 (Srigley et al., 2013). As a result of the meeting, tubulocystic RCC, acquired cystic disease associated RCC clear cell papillary RCC, and hereditary leiomyomatosis RCC syndrome-associated RCC were added to the classification as novel forms of RCC. In addition to this, translocation carcinomas were recognized as a family of tumors, rather than a single tumor type and t(6,11) translocation carcinoma was recognized as an additional member of this group.
The contemporary classification of RCC was formulated at the 2016 meeting of the WHO Renal Tumor Classification Panel (World Health Organization Classification of Tumours, 2016). This resulted in succinate dehydrogenase-deficient RCC being recognized as a distinctive form of RCC, while RCC-associated with neuroblastoma, which had been included in the 2004 WHO classification, was removed as a recognized entity, as it was considered that it is not a unified tumor type.
Since the publication of the Fourth Edition of the WHO Classification in 2016, a number of additional RCC tumor types have been described and of these multifocal oncocytoma-like tumors associated with oncocytosis (Warfel & Eble, 1982; Eble & Delahunt, 2018), eosinophilic solid and cystic RCC (Trpkov et al., 2017), biphasic squamoid alveolar RCC (Hes et al., 2016) and thyroid-like follicular renal cell carcinoma (Amin et al., 2009a) are likely to be included in the next iteration of the WHO renal tumor classification. The features of these novel tumor types are described in this review.
Multifocal oncocytoma-like tumors associated with oncocytosis
The occurrence of numerous tumors resembling oncocytoma was first reported in a single patient in 1982 and the diagnostic term oncocytomatosis was introduced, although more recently the term oncocytosis has been proposed (Warfel & Eble, 1982; Tickoo et al., 1999). The characterization of the renal lesions of the Birt-Hogg-Dubé syndrome has led to the understanding that this syndrome is associated with the development of numerous and bilateral oncocytic renal neoplasms (World Health Organization Classification of Tumours, 2016). It is now also acknowledged that these tumors may also arise de novo in those without constitutional features of Birt-Hogg-Dubé (Eble & Delahunt, 2018). Both the terms oncocytomatosis and oncocytosis have been used to describe situations where numerous eosinophilic tumors are present in one or both kidneys, although in the fourth edition of the WHO Renal Tumor classification the preference is for oncocytosis (World Health Organization Classification of Tumours, 2016). Currently there is confusion regarding the nature of oncocytosis and the term is used to describe kidneys with numerous, apparently sporadic oncocytomas as described in the 1982 case report, and to kidneys in patients having the manifestations of Birt-Hogg-Dubé. It is proposed that the term oncocytosis be confined to those tumors that occur sporadically and that the tumor complex be known as multifocal oncocytoma-like tumors associated with oncocytosis (Delahunt & Eble, 2005).
Reports of oncocytosis are limited and in studies to date the ages of patients range from 12 to 81 years with a slight male predominance (Eble & Delahunt, 2018). Oncocytosis is characterized by the presence of numerous oncocytic tumors – often numbering into the hundreds and even thousands, usually in both kidneys.
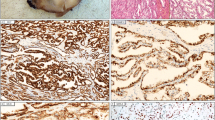
The renal tumors of oncocytosis usually consist of sheets and nests of cells with finely granular eosinophilic cytoplasm and a central nucleus showing low grade features (Figs. 1 and 2). Although these tumors resemble the oncocytic component of tumors seen in the Birt-Hogg-Dubé syndrome (which include chromophobe RCC and the so-called hybrid tumor, as well as oncocytoma), the tumors of onococytosis have genetic changes associated with typical oncocytoma, but not seen in Birt-Hogg-Dubé renal lesions (Klomp et al., 2010). The immunoexpression of oncocytosis does; however, differ from typical oncocytoma in that approximately 50% of tumors show diffuse positivity to cytokeratin 7 (Gobbo et al., 2010) - a feature associated with sporadic chromophobe RCC. Oncocytosis is frequently associated with progressive renal failure as tumors replace normal renal parenchyma (Farkas et al., 1999). This may be extensive and it is apparent that in many cases, what little normal parenchyma that remains is atrophic, due to compression by adjacent tumors. Despite the progressive nature of oncocytosis there is no evidence that these tumors have metastatic potential, although recurrence is common. In rare instances there have been associated papillary RCC and even clear cell RCC; however, this is not typical and it likely that these are incidental findings associated with end stage kidneys.
Eosinophilic solid and cystic renal cell carcinoma
Eosinophilic solid and cystic RCC (ESC RCC) was originally described in a series of female patients with tuberous sclerosis and was considered to be a distinctive renal tumor associated with the tuberous sclerosis complex (Guo et al., 2014). Subsequent studies showed that the tumor also occurred sporadically and like those tumors associated with tuberous sclerosis, the sporadic form also shows a strong female preponderance (Trpkov et al., 2016).
In a series of 16 cases from females with no personal or family history of tuberous sclerosis, patient’s ages ranged from 31 to 75 years (mean 57 years) (Trpkov et al., 2016). Grossly the tumors ranged up to 13.5cm in maximum extent although most were < 5 cm. The tumors were tan and had both cystic and solid areas macroscopically. In this series tumors were confined to the kidney, although more recently ESC RCC has demonstrated an ability to metastasize (McKenney et al., 2018). Histologically ESC RCC consists of cells often with strikingly voluminous eosinophilic and granular cytoplasm (Figs. 3 and 4). Multinucleate tumor giant cells are occasionally seen, while solid areas of tumor consist of sheets, nest or acini, usually with adjacent cystic areas - with the cyst wall consisting of aggregates of eosinophilic cells. Other typical features are intracytoplasmic vacuoles and psammoma bodies (Trpkov et al., 2016). Immunoexpression of these tumors shows some variability although cytokeratin 20, PAX 8 and vimentin are usually positive, CD10, racemase and epithelial membrane antigen can be equivocal, while cytokeratin 7 and CD117 are usually negative.
In an expansion of the original series incorporating ten new cases, it was noted that there were a variety of unique genetic changes with gains in 16p13.3-16q23.1, 7p21.2-7q36.2 and 13q14.2, while losses were seen at Xp11.21 and 22q11.23. Loss of heterozygosity was most frequently associated with loci of 16p, 11p, 9q and X (Trpkov et al., 2017). In this series gains were seen in TSC2 in 42% of cases, while loss of heterozygosity was seen in TSC1 in 33% of cases. In a separate series of cases, 8/9 pediatric tumors and 6/6 adult tumors were shown to have TSC1 or TSC2 mutations (Palsgrove et al., 2018). Interestingly it was noted that some of these tumors had atypical features; occurring in young patients, as well as showing multifocality and the ability to metastases – characteristics not seen in the earlier series. In this latter series morphological variations included a papillary architecture and chromophobe RCC-like morphology. In view of this it was claimed that these results expanded the morphologic spectrum of ESC RCC although immunohistochemical studies were limited, with most tumors showing cathepsin K positivity and focal, rather than diffuse, positivity for cytokeratin 20. More recently tumors showing some features in common with ESC RCC has been described from a series of 7 cases (Chen et al., 2019). These tumors showed TSC2 (2 cases) and MTOR (2 cases) mutations in the 5 cases studied, for which genetic data were available. Unlike ESC RCC, the series included male patients and the tumors had a predominantly nested morphology, being negative for cytokeratin 20. In common with ESC RCC these tumors did consist of cells with voluminous eosinophilic cytoplasm and intracytoplasmic vacuoles, and tumor giant cells were also present. The tumors showed positive cathepsin K, cytokeratin AE 1/3, epithelial membrane antigen and CD10 expression, while CD117 immunostaining was weak. While this latter group of tumors may represent a unique type of RCC, it is apparent that there is considerable variation in the morphology of tumors showing mutation of genes associated with tuberous sclerosis and the relationship between these tumors and more typical ESC RCC requires further detailed investigation.
Thyroid-like follicular renal cell carcinoma
Renal carcinomas showing morphological features similar to follicular carcinoma of the thyroid were first reported in a series of 6 cases (Amin et al., 2009b). Since that initial report the details of a further 33 cases have appeared in the literature, largely as case reports - with the largest series consisting of five cases (Eble & Delahunt, 2018; Chen et al., 2016). This entity was included in the ISUP Vancouver Classification of Renal Cell Neoplasia as an emerging/provisional entity. It was noted that while this probably represented a novel form of renal neoplasia, there was insufficient clinical and histological data to allow the inclusion of the tumor type in the formal classification of renal tumors (Srigley et al., 2013). A similar conclusion was reached at the meeting of the panel that formulated the renal tumor classification for the fourth edition of the WHO Classification of Tumours of the Urinary System and Male Genital Organs published in 2016 (World Health Organization Classification of Tumours, 2016).
Thyroid-like follicular RCC occurs in younger patients than the more common types of RCC, with the mean age of reported cases being 41 years (range 19–83 years). The tumor also occurs more commonly in females with the gender distribution of reported cases being female 25: male 14. For cases in which clinical details were available, 11 of patients were symptomatic at presentation, while in the remaining 27 cases the tumors were incidental findings (Eble & Delahunt, 2018). Interestingly, although there is no evidence of any association between these tumors and familial cancer syndromes, it has been noted that in 3 cases there was an association with leukemia/lymphoma (Eble & Delahunt, 2018).
The macroscopic features of thyroid-like follicular RCC are somewhat variable often being well circumscribed and ranging from tan/brown to grey in colour (Chen et al., 2016; Sterlacci et al., 2008). The tumors are usually small; however, the largest tumor reported to date was 13 cm in maximum extent. Areas of cyst formation, necrosis and/or hemorrhage are not uncommon, and foci of calcification may be present.
Histologically the tumors consist of follicles of variable sizes, composed of cuboidal to flattened cells, Often with eosinophilic cytoplasm and regular nuclei with prominent nucleoli (Srigley et al., 2013). The follicles frequently contain eosinophilic proteinaceous material and the overall morphologic features can be strikingly similar to follicular carcinoma of the thyroid (Figs. 5 and 6). Interestingly, nuclear grooving, a feature of thyroid follicular carcinoma, has only been rarely seen.
The immunoexpression of thyroid-like follicular RCC shows considerable variability for markers of renal neoplasia (CD10, RCC antigen, PAX 2 and PAX 8). The majority of tumors have been shown to be positive for cytokeratin 7, while all tumors are uniformly negative for TTF-1 and thyroglobulin. This latter feature permits distinction of these tumors from primary thyroid carcinoma which is of some importance as RCC metastatic to the thyroid, although uncommon, is well recognized. It also allows distinction between thyroid-like follicular RCC and follicular thyroid carcinoma metastatic to the kidney.
Early reports suggested that thyroid-like follicular RCC was not an aggressive tumor and in the initial series all tumors were organ-confined with no tumor recurrence (Amin et al., 2009b). More recently reports do indicate a malignant potential with metastases to the lungs (Sterlacci et al., 2008) and retroperitoneal nodes (Dhillon et al., 2011), meninges and skull (Dong et al., 2016), and lymph nodes (Chen et al., 2016) being reported from a total of four cases.
Biphasic squamoid alveolar renal cell carcinoma
Biphasic squamoid alveolar RCC, a tumor characterized by an unusual nested morphology and consisting of two distinctive cell types, was first reported as biphasic alveolo-squamoid renal carcinoma in 2012 (Petersson et al., 2012). This initial report was followed by a series consisting of 20 additional cases, published in 2016 in which the name of the tumor was changed to biphasic squamoid alveolar RCC (Hes et al., 2016). Since then several case reports and an additional series of 28 cases have been reported, with the suggested terminology again being altered, this time to biphasic papillary RCC (Troxell & Higgins, 2016; Trpkov et al., 2018).
In the two largest published series the ages of the patients at presentation ranged from 39 to 86 years and in one series the mean age was 55 years (Hes et al., 2016; Trpkov et al., 2018) Details relating to mode of presentation of these tumors are largely omitted from previous studies, although it has been noted that in two cases the tumors occurred in renal allografts (Troxell & Higgins, 2016).
As most studies are based on archival material, information relating to gross morphology is scant. In general the tumors may be white/grey and/or brown ranging up to 16 cm in maximum dimension, although most tumors were small, with the mean diameters from two series being 4.6 and 2.7 cm (Hes et al., 2016; Trpkov et al., 2018).
Biphasic squamoid alveolar RCC has distinctive histopathologic features consisting of two cell types. While a variety of architectural patterns have been described, these tumors typically consist of nests of cells with pale eosinophilic cytoplasm, and large nuclei with prominent nucleoli. Although keratin pearls have not been seen, these cells have been described as appearing squamoid and on ultrastructural examination have been shown to have desmosomes and tonofiliments (Petersson et al., 2012). These larger cells are usually surrounded by a layer of smaller cells with scanty cytoplasm, imparting a basophilic appearance to the lining of the nests of larger cells (Fig. 7). In some cases these tumors can show a striking resemblance to aggregates of glomeruli and it is not surprising that these tumors have been described as having a glomeruloid architecture (Fig. 8) (Trpkov et al., 2018; Mantoan Padilha et al., 2013). Variant morphologies have been reported, with tumors showing a compact pattern being included in series of so-called solid papillary RCC (Mantoan Padilha et al., 2013; Ulamec et al., 2016). Immunoexpression of these tumors can be helpful diagnostically as they show diffuse positive staining for pan-cytokeratin (cytokeratin AE1/AE3), cytokeratin 7, racemase, PAX8, epithelial membrane antigen and vimentin. CD10 immunostaining is variable, while the tumors are negative for carbonic anhydrase IX (Hes et al., 2016; Trpkov et al., 2018).
It has been suggested that biphasic squamoid alveolar RCC is related to type 1 papillary RCC as numerous tumors of both types have been reported in a single patient with familial MET mutation (Chartier et al., 2017). Some evidence of the inter-relationship between type 1 papillary RCC and biphasic papillary RCC is that both tumor types have been shown to have gains in both chromosome 7 and 17, although loss of heterozygosity in chromosomes 12, 20 and 21 has also been reported for biphasic squamoid alveolar RCC (Hes et al., 2016; Trpkov et al., 2018). Other types of RCC have also been reported in association with biphasic papillary RCC (Trpkov et al., 2018), although these are less commonly seen and probably represent co-incidental findings. As the association with papillary RCC is as yet unproven we prefer the term biphasic squamoid alveolar RCC for these tumors.
While most biphasic squamoid alveolar RCCs are organ confined at the time of diagnosis, being reported as pT1 stage category, metastatic spread has been noted. In reports where the diagnosis is unequivocal two patient died of disease, four were alive with extra-renal metastases and one had local tumor recurrence post-operatively, from a total of 37 patients for which follow-up data were available (Hes et al., 2016; Trpkov et al., 2018).
Conclusion
In this review we describe the features of the novel forms of renal cell carcinoma; multifocal oncocytoma-like tumors associated with oncocytosis, eosinophilic solid and cystic RCC, biphasic squamoid alveolar RCC and thyroid-like follicular renal cell carcinoma. These tumors are likely to be included in the next iteration of the WHO renal tumor classification.
References
Amin MB, Gupta R, Hes O et al (2009a) Primary thyroid-like follicular carcinoma-like tumor of the kidney. Report of 6 cases of a histologically distinctive adult renal epithelial neoplasm. Am J Surg Pathol 33:393–400
Amin MB, Gupta R, Hes O et al (2009b) Primary thyroid-like follicular carcinoma of the kidney. Report of 6 cases of a histologically distinctive distinctive adult renal epithelial neoplasm. Am J Surg Pathol 33:393–400
Chartier S, Méjean A, Richard S et al (2017) Biphasic squamoid alveolar renal cell carcinoma: 2 cases in a family supporting a continuous spectrum with papillary type 1 renal cell carcinoma. Am J Surg Pathol 41:1011–1012
Chen X, Dou FX, Cheng XB et al (2016) Clinicopathologic characteristics of thyroid-like follicular carcinoma of the kidney: an analysis of five cases and review of the literature. Chinese J Pathol 45:687–691
Chen Y-B, Mirsadraei L, Jayakumaran G et al (2019) Somatic mutations of Tsc2 or MTOR characterize a morphologically distinct subset of sporadic renal cell carcinoma with eosinophilic and vacuolated cytoplasm. Am J Surg Pathol 43:121–131
Delahunt B (1999) Sarcomatoid renal cell carcinoma. The final common dedifferentiation pathway of renal epithelial neoplasms. Pathology 31:185–190
Delahunt B, Cheville JC, Martignoni G et al (2013) The International Society of Urological Pathology (ISUP) grading system for renal cell carcinoma and other prognostic parameters. Am J Surg Pathol 37:1490–1504
Delahunt B, Eble JN (2005) History of the development of the classification of renal cell neoplasia. Clin Lab Med 25:231–236
Dhillon J, Tannir NM, Matin SF et al (2011) Thyroid-like follicular carcinoma of the kidney with metastases to the lungs and retroperitoneal lymph nodes. Hum Pathol 42:146–150
Dong L, Huang J, Huang L et al (2016) Thyroid-like follicular carcinoma of the kidney in a patient with skull and meningeal metastases. Medicine 95:e3314
Eble JN, Delahunt B (2018) Emerging entities in renal cell neoplasia: thyroid-like follicular renal cell carcinoma and multifocal oncocytoma-like tumours associated with oncocytosis. Pathology 50:24–36
Eble JN, Sauter G, Epstein J, Sesterhenn IA (eds) (2004) Pathology and genetics of tumours of the urinary system and male genital organs. World Health Organization classification of Tumours. IARS Press, Lyon
Farkas LM, Székely JG, Karátson A (1999) Bilateral, multifocal renal oncocytomasis with rapid progression leading to renal insufficiency. Nephrol Dial Transplant 14:2262–2263
Gobbo S, Eble JN, Delahunt B et al (2010) Renal cell neoplasms of oncocytosis have distinct morphologic, immunohistochemical, and cytogenetic profiles. Am J Surg Pathol 34:620–626
Guo J, Tretiakova MS, Troxell ML et al (2014) Tuberous sclerosis associated renal cell carcinoma: a clinicopathological study of 57 separate carcinomas in 18 patients. Am J Surg Pathol 38:1457–1467
Hes O, Condom Mundo E, Peckova K et al (2016) Biphasic squamoid alveolar renal cell carcinoma. A distinctive subtype of papillary renal cell carcinoma. Am J Surg Pathol 40:664–675
Klomp JA, Petillo D, Nierni NM et al (2010) Birt-Hogg-Dubé renal tumors are genetically distinct from other renal neoplasias and are associated with up-regulation of mitochondrial gene expression. BMC Med Genet 3:59
Kovacs G, Akhtar M, Beckwith BJ et al (1997) The Heidelberg classification of renal cell tumours. J Pathol 183:131–133
Mantoan Padilha M, Billis A, Allende D et al (2013) Metanephric adenoma and solid variant of papillary renal cell carcinoma: common and distinctive features. Histopathology 62:941–953
McKenney JK, Przybycin C, Trpkov K et al (2018) Eosinophilic solid and cystic (ESC) renal cell carcinomas have metastatic potential. Histopathology 72:1066–1067
Mostofi K, Davis CJ, Sobin LH et al (1998) World Health Organization international histological classification of Tumours. Histological typing of kidney Tumours. Second edition. Springer-Verlag, Berlin Heidelberg
Palsgrove DN, Yunjie L, Pratilas CA et al (2018) Eosinophilic solid and cystic (ESC) renal cell carcinoma harbor TSC mutations. Molecular analysis supports an expanding clinicopathologic spectrum. Am J Surg Pathol 42:1166–1181
Petersson F, Bulimbasic S, Hes O et al (2012) Biphasic alveolosquamoid renal carcinoma: a histomorphological immunohistochemical, molecular genetic, and ultrastructural study of a distinctive morphological variant of renal cell carcinoma. Ann Diagn Pathol 16:45969
Srigley JR, Delahunt B, Eble JN et al (2013) The International Society of Urological Pathology (ISUP) Vancouver classification of renal neoplasia. Am J Surg Pathol 37:1469–1489
Sterlacci W, Verdorfer I, Gabriel M et al (2008) Thyroid follicular carcinoma-like renal tumor: a case report with morphologic, immunophenotypic, cytogenetic, and scintigraphic studies. Virchows Arch 452:91–95
Stőrkel S, Eble JN, Adlakha K et al (1997) Classification of renal cell carcinoma. Cancer 80:987–989
Thoenes W, Störkel S, Rumpelt HJ (1986) Histopathology and classification of renal cell tumors (adenomas, oncocytomas and carcinomas). The basic cytological and histopathological elements and their use for diagnosis. Path Res Pract 181:125–143
Tickoo SK, Reuter VE, Amin MB et al (1999) Renal oncocytosis: a morphologic study of fourteen cases. Am Surg Pathol 23:1094–1101
Troxell ML, Higgins JP (2016) Renal cell carcinoma in kidney allografts: histologic types, including biphasic papillary carcinoma. Human Pathol 57:28–36
Trpkov K, Abou-Ouf H, Hes O et al (2017) Eosinophilic solid and cystic renal cell carcinoma (ESC RCC): further morphologic and molecular characterization of ESC RCC as a distinct entity. Am J Surg Pathol 4:1299–1308
Trpkov K, Athanazio D, Magi-Galluzzi C et al (2018) Biphasic papillary renal cell carcinoma is a rare morphologic variant with frequent multifocality: a study of 28 cases. Histopathology 72:777–785
Trpkov K, Hes O, Bonert M et al (2016) Eosinophilic, solid, and cystic renal cell carcinoma. Clinicopathologic study of 16 unique, sporadic neoplasms occurring in women. Am J Surg Pathol 40:60–71
Ulamec M, Skenderi K, Trpkov K et al (2016) Solid papillary renal cell carcinoma: clinicopathologic, morphologic, and immunohistochemical analysis of 10 cases and review of the literature. Ann Diagn Pathol 23:51–57
Warfel KA, Eble JN (1982) Renal oncocytomatosis. J Urol 127:1179–1180
World Health Organization Classification of Tumours (2016) In: Humphrey PA, Moch H, Reuter VE, Ulbright TM (eds) Pathology and genetics of the urinary system and male genital rgans, 4th edn. IARC Press, Lyon
Acknowledgements
None
Funding
None
Availability of data and materials
Not applicable
Author information
Authors and Affiliations
Contributions
The study was conceived and developed by all authors. BD, LE and HS wrote the manuscript. All authors contributed to the editing of the submitted draft. JNE provided additional illustrations. All authors have read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Delahunt, B., Eble, J.N., Egevad, L. et al. Emerging entities of renal cell neoplasia. Surg Exp Pathol 2, 10 (2019). https://doi.org/10.1186/s42047-019-0035-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s42047-019-0035-x