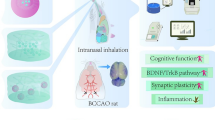
Abstract
Multi-infarct dementia (MID) is described as a chronic progressive decline in cortical cognitive function due to the occurrence of multiple infarcts in the cerebral vascularization throughout the gray and white matter. Current therapies of MID mostly focus only on slowing down MID progression and symptomatic medications. A novel therapy which is able to provide both preventive and curative properties for MID is of high interest. The purpose of this review is to identify the potential of Compound 21 (C21) gelatin nanoparticle through the nose-to-brain route as therapy for MID. C21, an angiotensin II type 2 receptor (AT2R) agonist, has shown to reduce the size of cerebral infarct in rodent models, resulting in the preservation and improvement of overall cognitive function and prevention of secondary neurodegenerative effects. It is also shown that C21 decreases neuronal apoptosis, improves damaged axons, and encourage synapse development. The challenge remains in preventing systemic AT2R activation and increasing its low oral bioavailability which can be overcome through nose-to-brain administration of C21. Nose-to-brain drug delivery of C21 significantly increases drug efficiency and limits C21 exposure in order to specifically target the multiple infarcts located in the cerebral cortex. Adhering C21 onto gelatin nanoparticles may enable longer contact time with the olfactory and the trigeminal nerve endings, increasing the potency of C21. In summary, treatment of C21 gelatin nanoparticle through nose-to-brain delivery shows high potential as therapy for vascular dementia. However, clinical trials must be further studied in order to test the safety and efficacy of C21.
Similar content being viewed by others
Introduction
The multitude of neurological disorders with considerable cognitive deterioration in one or more cognitive domains (executive, memory, language, and visuospatial) falls under the general term major neurocognitive disorder, often known as dementia. The DSM-5 diagnosis of major neurocognitive disorder requires independence impairment in daily tasks and is not better described by another mental illness [1]. The four common types of dementias are Alzheimer’s disease, vascular dementia, Lewy body dementia and frontotemporal dementia [2]. Vascular dementia is caused by neuronal oxygen deprivation that restrict blood supply to the brain. As a result of ischemic tissue damage in a specific area of the brain, infractions cause substantial cognitive impairment such as confusion, disorientation, trouble speaking, comprehending speech, and visual loss [3, 4]. Vascular dementia causes an estimation of 17.9% of dementia cases and is the second most prevalent type of dementia after Alzheimer’s disease [5]. Globally, estimates of vascular dementia prevalence range from 0.9 to 3.3 percent [6]. In underdeveloped nations, these estimates range from 0.7 to 2.1 percent of individuals over the age of 55 [7]. Several subtypes of vascular dementia include small vessels disease (subcortical infarct), multi-infarct dementia (cortical infarct), strategic dementia, hypoperfusion dementia, mixed dementia, hemorrhagic dementia and hereditary vascular dementia [8]. The spectrum of clinical vascular cognitive impairment corresponds with the specific area and extent of deterioration [9]. The heterogeneity in anatomic and neurological alterations in vascular cognitive impairments is important in pathogenic processes that affect neurocognitive performance, emotional range, and physiological functionality.
The most prevalent subtype of vascular dementia is estimated to be multi-infarct dementia (MID) or also known as cortical infarct dementia [10]. MID involves the cortical cognitive impairment brought on by numerous infarcts across both white and gray matter in the cerebral arterioles post-occlusions [11]. As MID is most associated with cortical infarct, executive functioning is most affected, which is then followed by memory difficulties, and emotional integrity problems, compared to other subtypes of vascular dementia [12]. MID is linked to cortical cerebrovascular disorders such as stroke, lacunar infarcts, atherosclerotic conditions, such as diabetes, hypertension and coronary heart disease [13]. Demographic factors such as sex, age, low education, family medical history of dementia, prior cognitive decline, premorbid diseases such as diabetes, atrial fibrillation, stroke, hypertension and dyslipidemia are among the predictors of MID [14].
MID has no known definitive treatment since the brain damage is irreversible [15]. Current therapeutic approaches in MID are similar to that of other subtypes of vascular dementia. Treatment aiming to slow down MID progression and symptomatic medications such as cholinesterase inhibitors (donepezil, rivastigmine, galantamine) and memantine are currently used [16]. In a recent meta-analysis study, donepezil and galantamine showed improved Alzheimer’s Disease Assessment Scale-Cognitive Subscale (ADAS-cog) in vascular dementia, but not rivastigmine [17]. The use of cholinesterase inhibitors was linked to a twofold increase in the likelihood of cessation due to side-effects such as gastrointestinal disorders, symptomatic bradycardia, agitation, anorexia, insomnia, leg cramps, nausea, nervous system impairments and vomiting [17, 18]. Alongside, although memantine has a slight positive impact in patients with severe vascular dementia, it is likely no better than placebo in persons with mild vascular dementia [19]. Elderly frequently have concurrent renal impairment, which is an important precaution factor in the use of memantine [20]. Moreover, studies showed that an imbalance between inflammatory mediators was notably apparent in MID patients when compared to controls and other subtypes of dementias, with an elevation in TNF and a decline in IL-10 production [21, 22]. This suggests the idea of modulating pro and anti-inflammatory cytokine as a potential therapeutic method. Future neuroprotective medicines are predicted to be effective in the treatment of MID until further research is done.
A recent finding shows that direct angiotensin II type 2 receptor (AT2R) activation reduces inflammation and mitigates ischemic vascular dementia caused by hypoperfusion [23, 24]. Correlating to previous findings, an AT2R agonist namely Compound 21 (C21) is reported to prevent cognitive decline, preserved reference memory, improved cognitive flexibility and neurovascular advantages without the risk of unexpected severe side-effects by means of cytokine modulation [23, 25]. The challenge in preventing AT2R activation across the body and low oral bioavailability of C21 can be overcome by limiting the exposure through nose-to-brain administration [26]. Nose-to-brain administration of C21 is found to not affect blood pressure and heart rate [27]. Furthermore, the use of mucoadhesive agent gelatin enables longer contact time of the compound to deliver the effects through olfactory bulb and trigeminal nerves passageway [28, 29]. Additionally, research on mucoadhesive agent found that neural drugs can be successfully transported into the brain to display neuro-recovery function due to the properties of mucoadhesive agent gelatin nanoparticle through the nose-to-brain route [30]. Thus, the use of C21 and gelatin nanoparticle with nose-to-brain administration route could be a promising and feasible way to treating MID.
Following the success in recent discoveries, the authors are interested in investigating further regarding this modality so that it can provide better prospects in the management of MID.
Multi-infarct dementia pathophysiology
The central involvement of cerebral blood vessels in the wide range of cognitive impairment diseases emphasizes the significance of vascular structure and function [31]. The brain demands a constant and well-regulated flow of blood due to its high energy consumption and lack of fuel reserves [31, 32]. Given the critical necessity of the cerebral blood supply for the anatomical and functional integrity of the brain, changes in cerebral blood arteries have a significant influence on cognitive performance [11]. Microangiopathy and macroangiopathy of the cerebral arteries have similar risk factors and pathological characteristics in the development of vascular dementia [33, 34]. Microangiopathy is predominantly caused by lipohyalinosis and microatheroma that cause thromboembolism which leads to vessel stenosis as well as cerebral ischemia [35]. Correspondingly, macroangiopathy is caused mostly by atherosclerosis, which manifests itself as a series of ischemia episodes that damage brain networks over time [36]. Both pathways of vessel damage eventually lead to vascular dementia [11, 13]. Furthermore, high blood pressure, cholesterol and smoking all raise the risk of vascular dementia [37].
Local intersecting thrombotic processes connected with hypertensive intracranial hemorrhage are examples of pathophysiological primary central nervous system (CNS) vascular impairment. Vascular bleeding caused by the rupture of an intracranial aneurysms or an arteriovenous malformation may occur. Atypical cerebrovascular pathologies including inflammation and vascular dissection may also be present [38]. Connective tissue disorders, viral causes, oncogenic causes, prothrombotic diseases, clotting disorders and electrolyte imbalance are examples of systemic factors. Typically, atherosclerosis progresses with age as well as potential risks such smoking, genetic predisposition, hypertension, diabetes mellitus and dyslipidemia. The eventual outcome is vascular occlusion. Microvascular damage, including lacunar infarction and other SVD elements, is most often linked to chronic hypertension on intracranial vasculature which culminate in a gradual occlusive illness with fibrotic necrosis and lipohyalinosis [8, 39,40,41]. Hypoperfusion and thromboembolic disorders cause hypoxic conditions, oxidative stress and inflammatory reactions in addition to lowering cerebral blood flow (CBF). Disruption to vascular epithelium, microglial and neurons leads to neurovascular separation and additional reductions in CBF. Oxidative stress also disrupts the balance of antioxidants to reactive oxygen species (ROS). Cerebral hypoxia may cause apoptosis, vasculature malfunction, blood–brain barrier (BBB) leakage, endothelial dysfunction, and neuro-inflammatory reaction [42].
The formation of vascular-mediated cognitive decline represents distinct regions of complex cognitive interaction from a neurobiological viewpoint (Fig. 1).
Compared to other subtypes of vascular dementia, an increase of pro-inflammatory cytokines (TNF) and a decrease of anti-inflammatory cytokines (IL-10) in cases of MID are observed [43]. The neurological presentations of MID may be caused by cumulative effects of both large vessel occlusions such as atherothrombosis and cardioembolism or accumulated cerebral small vessel disease (SVD) such as lipohyalinosis [8, 43, 44].
Consequently, large vessel occlusions and accumulated SVD result in the decrease of CBF which leads to cerebral hypoxia and eventually impairment of cognitive function, specifically the executive function. The severity of the cognitive impairments varies depending on the degree of neurological effects caused by MID [12]. Manifestations of MID also vary based on the methodology of the cerebrovascular blockage, the extent of neuronal loss, the influence on neurological signal transduction interoperability, the implications on specific brain regions, as well as distant effects [8, 41]. Intracranial arteries and the middle cerebral artery are the most common blood vessels associated with MID. Atherosclerosis is the most commonly found vascular disease in cases of MID [45].
Compound 21 overview
Compound 21 is an orally active AT2 receptor agonist which is non-peptidergic and highly selective. C21 allows selective stimulation of AT2 receptors under any experimental conditions. Through pharmacological approaches, the agonist concentrations can be increased at the receptor site exceeding the natural ligand, angiotensin II. It could be done without compromising selectivity. AT2 receptor-mediated actions can be exposed in a given tissue even under conditions of low receptor expression [46]. The anti-inflammatory effects of C21 provide a therapeutic tool to intercede the processes of diseases such as myocardial infarction, stroke and reduce inflammatory reaction is dermatological disease. Moreover, C21 possess neuroprotective and regenerative characteristics that are beneficial to treat the sequelae of damage in the nervous system [46]. Another research found that oral administration of C21 has beneficial effects on delaying brain damage and protective impacts towards neuronal tissue from ischemic damage [47]. Independent of its initial neuroprotective benefits, C21 treatment can prevent gradual post-stroke cognitive deterioration in young, aged and diabetic male rats. C21 shows anti-inflammatory effects on Parkinson’s disease and impressive blood–brain barrier penetration in mice model [48].
Previous studies have shown that C21 has positive impacts in patients diagnosed with vascular dementia [49]. MID is caused by a decrease in CBF and is characterized by changes in white matter. An experiment demonstrated that direct AT2 receptor stimulation by C21 enhanced cognitive decline in Bilateral Common Carotid Artery Stenosis (BCAS) mouse model. It is caused by the reduction of CBF and a decrease in inflammatory cytokine expression [23]. Another study reported that treatment with C21, enhanced cognitive decline in a mouse with Alzheimer’s disease model by increasing CBF and the outgrowth of neurite. It was also reported that C21 promotes neurite elongation in primary cultured hippocampal neurons [50]. C21 can be expected to protect against nerve damage and promotes neuronal development. Treatment with C21 has also been reported to increase vascular density after stroke. With this ability to increase CBF and reduce inflammation, direct AT2 stimulation by C21 could prevent ischemic MID. Direct AT2 receptor stimulation is expected to be a new therapeutic strategy for the prevention and treatment of MID.
Gelatin nanoparticles
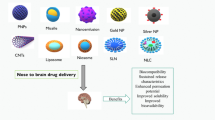
Due to the submicron size, nanoparticles have a potential in improving the efficacy and potency of drugs. NPs have surfaces that are able to modify specific ligands, functional groups and polymers of different sizes in order to increase the specificity towards target sites. Gelatin is a kind of protein obtained from the hydrolysis of collagen [51]. It is derived from natural sources such as collagen by acid or alkaline hydrolysis [52]. Gelatin has also been used as hemostatic agent for years [53]. There are two types of gelatin, which are type A and type B. The difference of type A and B is on the iso-electric points (IEPs). Type A has IEP 7–9 and type B has IEP 4–5. In addition, different types of gelatin also have different drug release potential. Type B gelatin nanoparticles (GNP) have been shown to have better drug delivery potential than type A. GNP is very promising in drug delivery as it is nontoxic, low cost, easy availability, biodegradable and bioactive. The nanoparticles have been used in many applications for therapy, such as cancer therapy, tissue engineering devices and diagnostics. GNPs are very suitable on reducing toxicity that is associated with most drugs. Nanoparticles also have the ability to penetrate the blood brain barrier. The BBB has a strong and complex physiological structure, but with GNPs’ small sizes and perfect surface functionalization they can easily penetrate their walls to reach site of action [51].
Therapeutic biological particles smaller than 300 nm in size have the capability to bypass the BBB and directly enter the brain via the cribriform plate to perform their therapeutic functions. This particles can gain access to the CNS through an intranasal administration [54]. Intranasal administration of CNS-acting drugs may improve their pharmacokinetic and pharmacodynamic profiles. [55]. The particles enter the brain in two ways: olfactory and trigeminal pathways. [55]. The residence time at the delivery site is limited due to the mucociliary clearance mechanism, which may reduce the concentration level in the CNS. Since gelatin nanoparticles have a higher negative charge, they may reduce mucociliary clearance, increase residence time at the site of administration and enhance therapeutic effects when administered intranasally [54].
Pharmacodynamic of Compound 21
C21 affects blood vessel endothelium dilatation, inflammatory reaction inhibition, and neuronal repair and regeneration. In addition, treatment with C21 has proven to increase the number of synapses, improve impaired axons, and synapse development [56]. In a study, it was found from the measurements of MRI, that chronic treatment with C21 may delay the onset of brain abnormalities. Other than that, anti-inflammation effects of C21, such as IL-6 and TNFα downregulation appear to be an important mechanism of action for C21 in providing neuroprotection [57].
C21 treatment results in an increase of CBF as assessed by laser speckle flow cytometry and hippocampal field-excitatory postsynaptic potential. Activation of AT2 receptors stimulates nitric oxide (NO)/cyclic guanosine monophosphate (cGMP) release and may indirectly mediate vascular relaxation and blood flow through the regulation of bradykinin release. CBF collectively functions as part of the neurovascular unit in maintaining homeostasis of the cerebral microenvironment. C21 also may induce cognitive enhancement, which is attenuated by the co-administration of icatibant, a bradykinin B2 receptor antagonist. As a result, direct activation of AT2 receptors enhances spatial learning due to an increase in microcirculation, partly through the regulation of bradykinin [58].
Pharmacokinetics of Compound 21 nose-to-brain
In order to bypass the blood–brain barrier and avoid hepatic first-pass metabolism, C21 is administered through the nose-to-brain route [59, 60]. The C21 is specifically administered into the upper nasal cavity. It will then be absorbed and travel towards the brain through either three of the following pathways–the olfactory nerve, the trigeminal nerve, or the vascular pathway, where the compound will be distributed, entering the cortex of the brain and the brainstem. In a study on ischemic stroke rats, C21 reaches the affected cerebral cortex and striatum 30 min after administration through the nose-to-brain route [27].
There are two general transportation mechanisms of drugs and peptides into the brain from the upper nasal cavities. The first one is intraneuronal transport, where the drug or peptide is taken up into the olfactory neuron terminals by endocytic and receptor-mediated mechanisms. This transport mechanism requires hours to days to reach the targeted site of therapy. The second mechanism is extra neuronal transport, where the agent penetrates the brain through intercellular clefts and travels along a diffusion gradient along the outside of the axons. This mechanism allows the agent to reach the brain up to within a minute [27].
Construction and administration of gelatin nanoparticles
There are various methods to prepare gelatin nanoparticles that has been developed over the years. Some of these methods are two-step desolvation, simple coacervation or ionic gelation, oil-in-water emulsion, reverse phase preparation, microemulsion, inverse microemulsion, nanoprecipitation, modified desolvation technique, and self-assembly through chemical processes and mixing [61,62,63].
Two-step desolvation
Two-step desolvation method is the most commonly used method to prepare GNPs. The essence of the procedure is to add a desolvating agent such as ethanol, alcohols, or acetonitrile to an aqueous gelatin solution under certain controlled conditions [63]. The solution is then heated and steadily stirred at 40 °C [64]. The dehydrated solution will then be separated between particles with low (< 65kDA) and high molecular weight [51, 61]. The low molecular weight particles will be discarded through decantation to increase the stability and the homogenous size of the particles [51]. While the higher weight particles are dissolved into distilled water. The pH is then lowered to minimize the size of particles formed [65]. Then a desolvating agent is slowly added before Glutaraldehyde (GA) is immediately added as well [51, 61, 63, 65, 66]. The advantage of this method is that it produces homogenous-sized GNPs. The disadvantages are that it has low yield and reproducibility from the first step and it requires specific conditions such as pH, temperature, and molecular weight of the particles for maximized yield, making the procedure relatively complex [63, 67].
Simple coacervation
This method of preparation relies on the ability of polyelectrolytes to undergo cross-linkage when oppositely charged ions are introduced [61, 66]. A coacervating agent is added into the gelatin solution which separates it into two layers [61, 63]. GA is then added as a cross-linking agent to form GNPs. The disadvantages of this method are that the particles tend to aggregate, high polydispersity index (PDI), and relatively large GNPs [63].
Water-in-oil emulsion method
The gelatin solution, loaded with the drug, in this case C21, is emulsified to form a water-in-oil emulsion. Then, a cross-linking agent, GA, is added and then added to ethyl acetate to form stable GNPs [61, 66]. The GNPs are then washed using centrifugation method and are resuspended in acetone and buffer [68]. The advantage of this method is that the procedure is straightforward and simple to execute. However, the resulting GNPs tend to aggregate and have rough surfaces [66].
Modified desolvation
The modified desolvation method utilizes the principles of thermodynamics to allow self-assembly of the GNPs [66]. This method is a relatively straightforward, fast, and inexpensive procedure. The main advantage of using this modified desolvation method compared to the conventional two-step desolvation method is that any bloom valued gelatin can be used to construct GNPs [67]. The GNPs produced through this method is also relatively homogenous in size and has a high yield (up to 95%) when isopropyl alcohol is used as the desolvating agent [63, 67]. Figure 2 shows the illustrated method which presents a simpler procedure to construct gelatin nanoparticles compared to the conventional desolvation method, as described by Khramtsov et al.
Isopropyl alcohol is used over methanol and ethanol since it produces a higher number of homogenous particles in the suspension with less volume [67]. A solution of gelatin B, 75 bloom (10 mg/mL, pH 11), is mixed with isopropyl alcohol. The suspension will then be put into water bath for 30 min at an optimal temperature of 40 °C and < 65 °C to avoid denaturation [61, 67]. GA is then added to stabilize the suspension before it is centrifugated, combined to increase its concentration, and finally sonicated on ice. Higher yields and smaller-sized GNPs can be obtained through increasing the volume of isopropyl alcohol added or lowering the pH [67].
Loading of C21 into GNPs
The next step is to load C21 into the GNPs through two methods depending on the affinity of the drug to the GNPs. The first method allows the GNPs to be soaked in a solution of C21 and the GNPs are loaded through adsorption [61]. To maximize its entrapment efficiency, the GNP has to be loaded near to its isoelectric point [62]. The second method is to incorporate C21 into the gelatin solution before the preparation of GNPs [61].
Sterilization of GNPs
The GNPs then undergo autoclave sterilization to eliminate potentially harmful contamination from microorganisms. This method done under mild conditions has been reported to increase the thermal resistance of the GNPs. Nonetheless, autoclave sterilization poses some disadvantages such as increased break down and aggregation, lowered turbidity, decreased degree of cross-linkage, and increased content of free amino group [67].
Administration of C21-loaded GNPs
There are three main pathways in which GNPs can be administered through intranasal delivery, passing the BBB [69]. These pathways are the olfactory nerve, the trigeminal nerve, and the vascular pathway [70]. Nose-to-brain delivery can be done by first depositing the GNPs into the posterior and upper nasal cavity using devices that enable effective deposition such as breath-actuated nasal sprays, electronic atomizer, colloidal formulations, and powder-based exhalation delivery system, or simpler devices such as dropper or a needleless syringe [70,71,72]. Nanoparticles of different sizes travel towards the CNS through different pathways. Nanoparticles around 100 nm in size can be delivered through the trigeminal or the olfactory nerve. Meanwhile, larger nanoparticles can only be delivered through the former pathway [69]. Studies have also shown that nanoparticles over the size of 900 nm are unable to pass the BBB [72].
Following this information, most pre-clinical studies on animals have used devices which can maximize delivery through these pathways. A rat intranasal catheter is inserted into the nostril in order to reach the upper nasal cavity, specifically the cribriform plate, and deliver the drug through the olfactory nerve pathway. After 60 s, the intranasal catheter is removed [27]. In another animal study, a plastic pipette was used to administer GNPs intranasally on mice [73]. Another study uses gel loading pipette tips to administer nanoparticles suspended in saline [74].
Neurovascular regeneration of angiotensin II type 2 receptor activation
Some clinical researchers have found the beneficial effects of C21 in vivo as an angiotensin II type 2 receptor agonist in regard to neural rejuvenation shown in Table 1. The activation of angiotensin II type 2 receptors appears to be neuroprotective, as it encourages vasodilation, enhances cerebral microcirculation, lessen oxidative stress, reduces inflammation (both by decreasing the expression of pro-inflammatory cytokines and increasing the expression of anti-inflammatory mediators) and provides protection against brain damage [79]. In a neurovascular study, angiotensin II promotes cerebrovascular remodeling, increases vascular inflammation and oxidative stress, and affects CBF [80]. In regard to its anti-inflammatory, antiproliferative and tissue-regenerating characteristics, AT2R activation has emerged as a new treatment approach in vascular and central nervous system disorders [50, 81,82,83].
AT2Rs are G protein-coupled receptors that are found on neurons, astrocytes, and microglia in the cortex, hippocampus, locus coeruleus, basal ganglia, and blood vessels [84]. AT2R activation increases the synthesis of cGMP, NO, and bradykinin, which helps to mediate vasodilation [85, 86]. The rapid generated temporary vasodilation mediated by neuronal NO guarantees that CBF changes are adequate to fulfill metabolic expenditure needs [87]. Activation of AT2Rs by the selective AT2R agonist increased CBF and showed anti-inflammatory as well as antioxidant properties, likely by minimizing inflammation and lowering ROS production [50, 88, 89]. Furthermore, activation of the AT2R is found to increase the expression of MMS2, a DNA repair factor, as well as the activity of Src homology 2 domain-containing protein-tyrosine phosphatase 1 (SHP-1) [90]. MMS2 binding to ubiquitin-conjugating enzyme 13 (Ubc13) is required to activate DNA repair pathway in DNA damage response [91]. Ubc13 activation has been shown to be required for pre-synaptic synapse formation, subsequent synaptic growth and development, long-term memory, and neural development (Fig. 3) [92].
Clinical effects of C21-loaded GNPs
Although currently, clinical trials of the safety and efficacy of the use C21 on human patients with vascular cognitive impairment are still on phase 1 or 2, C21 has shown to be safe to use in humans with a dose of ≤ 100 mg [83]. Results on animal studies have also shown positive impacts in cognitive functions and memory after treatment with C21 [25, 83]. A pre-clinical study has shown that when C21 is administered over a prolonged amount of time, it can maintain overall cognitive function [78]. The drug has also shown to provide vascular protection and BBB preservation by increasing the expression of occludin, claudin-5, and Zonula occludens-1 (ZO-1) [83].
Studies on the clinical effects of C21-loaded GNPs are still very limited. However, studies on animals have presented promising results for the use of C21 in humans using other methods of drug delivery, such as oral, intraperitoneal, and injections. C21 has shown to mediate neuroprotection through AT2R stimulation [27, 76]. C21 also promotes the endothelial NOS (eNOS) pathway thus activating eNOS enzymes which has been proven to increase levels of IL-10 as neuroprotective cytokine and brain-derived neutrophic factor (BDNF) with no effect on the blood pressure. C21 also decreases ischemia-induced nitrative stress and on the other hand, increases vascular density in the ischemic hemisphere [76, 93]. Another study on hypertensive rats suggests that treatment with C21 improves motor deficit [47]. Other than that, C21 stimulates the AT2R, which in turn maintains or increases the CBF and stimulate increase in stem cell proliferation [27, 94]. In another study, treatment with C21 has proven to significantly decrease neuronal apoptosis in the cortex, hippocampus, amygdala, and hypothalamus. The same study also suggests that C21 improves repair of damaged axons and development of synapses in the brain [56]. These results support the potential use of C21 in treating MID in humans through its neuroprotective and restorative properties.
Another animal study investigates the ability of C21 treatment to prevent decrease in spatial learning. Results show decrease in cognitive decline in rodent models after treatment with C21 [23]. Another study shows that AT2R expression is more prominent in female mice due to the presence of endogenous estrogen. Thus, selective treatment of C21 throughout the estrous cycle provides a positive impact on the improvement of neurological function in female mice compared to male mice [95]. Interestingly, another study reports that treatment of C21 does not improve cognition in female rats [96]. Another study also supports the previously mentioned findings, which is that C21 treatment has proven to decrease size of cerebral infarct and neurological deficits in rodent models [97]. Chronic treatment of C21 has also proven to have anti-inflammatory effects [89]. A recent animal study shows that delayed administration of C21 results in preservation of brain tissue volume and improvement in myelination after stroke, which shows that C21 is also beneficial in reducing or preventing secondary neurodegenerative processes [77]. Another study suggests that C21 may improve stroke recovery by decreasing the ratio of pro-/anti-inflammatory microglia ratio [96]. Furthermore, one more study has proven that C21 has arteriogenic and angiogenic properties [49]. Negative side-effects of C21 are still not well-studied yet. Overall, these studies lead towards promising results for the use of C21 on the preservation and improvement of cognition, although further clinical studies must be conducted to investigate its safety and effectiveness in patients.
Conclusion
Based on the review of literature, C21 has been proven to provide neuroprotection, restoration of axons, and preservation of cognition post-stroke. Nose-to-brain drug delivery of C21 GNP significantly increases drug efficiency and contact time with nerve endings of the olfactory and trigeminal nerves by targeting administration on the upper and posterior nasal cavity through intranasal administration. Treatment of C21 through nose-to-brain delivery shows high potential for use in the therapy for MID.
Availability of data and materials
Not applicable.
Abbreviations
- ADAS-cog:
-
Alzheimer’s Disease Assessment Scale-Cognitive Subscale
- AT1 19AT2R:
-
Angiotensin II type 2 receptor
- BBB:
-
Blood–brain barrier
- BCAS:
-
Bilateral common carotid artery stenosis
- CBF:
-
Cerebral blood flow
- cGMP:
-
Cyclic guanosine monophosphate
- C21:
-
Compound 21
- CNS:
-
Central nervous system
- GA:
-
Glutaraldehyde
- GNP:
-
Gelatin nanoparticles
- IEP:
-
Iso-electric point
- MMS2:
-
DNA repair factor
- NO:
-
Nitric oxide
- PDI:
-
Polydispersity index
- ROS:
-
Reactive oxygen species
- SHP-1:
-
Src homology 2 domain-containing protein tyrosine phosphatase 1
- SVD:
-
Small vessel disease
- Ubc13:
-
Ubiquitin-conjugating enzyme 13
- ZO-1:
-
Zonula occludens-1
References
Association AP, Force APAD-5 T (eds). Diagnostic and statistical manual of mental disorders : DSM-5. Arlington: American Psychiatric Association, 2013.
Duong S, Patel T, Chang F. Dementia: what pharmacists need to know. Can Pharm J (Ott). 2017;150:118–29.
Vijayan M, Reddy PH. Stroke, vascular dementia, and Alzheimer’s disease: molecular links. J Alzheimers Dis. 2016;54:427–43.
Gottesman RF, Schneider ALC, Zhou Y, Coresh J, Green E, Gupta N, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA. 2017;317:1443–50.
Asada T. Prevalence of dementia in Japan: past, present and future. Rinsho Shinkeigaku. 2012;52:962–4.
Rizzi L, Rosset I, Roriz-Cruz M. Global epidemiology of dementia: Alzheimer’s and vascular types. Biomed Res Int. 2014;2014: 908915.
Yeverino-Castro SG, Mejía-Arango S, Mimenza-Alvarado AJ, Cantú-Brito C, Avila-Funes JA, Aguilar-Navarro SG. Prevalence and incidence of possible vascular dementia among Mexican older adults: analysis of the Mexican Health and Aging Study. PLoS ONE. 2021;16:1–15.
Bir SC, Khan MW, Javalkar V, Toledo EG, Kelley RE. Emerging concepts in vascular dementia: a review. J Stroke Cerebrovasc Dis. 2021. https://doi.org/10.1016/j.jstrokecerebrovasdis.2021.105864.
Dichgans M, Leys D. Vascular cognitive impairment. Circ Res. 2017;120:573–91.
Thal DR, Grinberg LT, Attems J. Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol. 2012;47:816–24.
Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–66.
Al-Adawi S, Braidy N, Essa M, Al-Azri F, Hussain S, Al-Sibani N, et al. Cognitive profiles in patients with multi-infarct dementia: an Omani study. Dement Geriatr Cogn Dis Extra. 2014;4:271–82.
Gorelick PB, Scuteri A, Black SE, DeCarli C, Greenberg SM, Iadecola C, et al. Vascular contributions to cognitive impairment and dementia. Stroke. 2011;42:2672–713.
Baskys A, Cheng J. Pharmacological prevention and treatment of vascular dementia: approaches and perspectives. Exp Gerontol. 2012;47:887–91.
Dementias: Hope Through Research. 2017. https://doi.org/10.1016/S0140-6736(02)11667-9.
Wang H-F, Yu J-T, Tang S-W, Jiang T, Tan C-C, Meng X-F, et al. Efficacy and safety of cholinesterase inhibitors and memantine in cognitive impairment in Parkinson’s disease, dementia, and dementia with Lewy bodies: systematic review with meta-analysis and trial sequential analysis. J Neurol Neurosurg & Psychiatry. 2015;86:135–43.
Chen Y, Zhang J, Wang Y, Yuan J, Hu W. Efficacy of cholinesterase inhibitors in vascular dementia: an updated meta-analysis. Eur Neurol. 2016;75:132–41.
Birks J, McGuinness B, Craig D. Rivastigmine for vascular cognitive impairment. Cochrane database Syst Rev 2013; CD004744.
McShane R, Westby MJ, Roberts E, Minakaran N, Schneider L, Farrimond LE, et al. Memantine for dementia. Cochrane Database Syst Rev. 2019. https://doi.org/10.1002/14651858.CD003154.pub6.
Moritoyo T, Hasunuma T, Harada K, Tateishi T, Watanabe M, Kotegawa T, et al. Effect of renal impairment on the pharmacokinetics of memantine. J Pharmacol Sci. 2012;119:324–9.
Custodero C, Ciavarella A, Panza F, Gnocchi D, Lenato GM, Lee J, et al. Role of inflammatory markers in the diagnosis of vascular contributions to cognitive impairment and dementia: a systematic review and meta-analysis. GeroScience. 2022;44:1373–92.
Saito H, Togashi H, Yoshioka M, Nakamura N, Minami M, Parvez H. Animal models of vascular dementia with emphasis on stroke-prone spontaneously hypertensive rats. Clin Exp Pharmacol Physiol Suppl. 1995;22:S257–9.
Iwanami J, Mogi M, Tsukuda K, Wang X-L, Nakaoka H, Kan-no H, et al. Direct angiotensin II type 2 receptor stimulation by compound 21 prevents vascular dementia. J Am Soc Hypertens. 2015;9:250–6.
Balogh M, Aguilar C, Nguyen NT, Shepherd AJ. Angiotensin receptors and neuropathic pain. Pain reports. 2021;6:e869–e869.
Ahmed HA, Ishrat T, Pillai B, Bunting KM, Vazdarjanova A, Waller JL, et al. Angiotensin receptor (AT2R) agonist C21 prevents cognitive decline after permanent stroke in aged animals—a randomized double- blind pre-clinical study. Behav Brain Res. 2019;359:560–9.
Verdonk K, Durik M, Abd-Alla N, Batenburg WW, van den Bogaerdt AJ, van Veghel R, et al. Compound 21 induces vasorelaxation via an endothelium- and angiotensin II type 2 receptor-independent mechanism. Hypertension. 2012;60:722–9.
Bennion DM, Jones CH, Dang AN, Isenberg J, Graham JT, Lindblad L, et al. Protective effects of the angiotensin II AT(2) receptor agonist compound 21 in ischemic stroke: a nose-to-brain delivery approach. Clin Sci (Lond). 2018;132:581–93.
Djupesland PG, Messina JC, Mahmoud RA. The nasal approach to delivering treatment for brain diseases: an anatomic, physiologic, and delivery technology overview. Ther Deliv. 2014;5:709–33.
Pathak R, Prasad Dash R, Misra M, Nivsarkar M. Role of mucoadhesive polymers in enhancing delivery of nimodipine microemulsion to brain via intranasal route. Acta Pharm Sin B. 2014;4:151–60.
Zhao Y-Z, Jin R-R, Yang W, Xiang Q, Yu W-Z, Lin Q, et al. Using gelatin nanoparticle mediated intranasal delivery of neuropeptide substance P to enhance neuro-recovery in hemiparkinsonian rats. PLoS ONE. 2016;11: e0148848.
Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, et al. Vascular cognitive impairment and dementia: JACC scientific expert panel. J Am Coll Cardiol. 2019;73:3326–44.
Mergenthaler P, Lindauer U, Dienel GA, Meisel A. Sugar for the brain: the role of glucose in physiological and pathological brain function. Trends Neurosci. 2013;36:587–97.
Li Q, Yang Y, Reis C, Tao T, Li W, Li X, et al. Cerebral small vessel disease. Cell Transplant. 2018;27:1711–22.
Okroglic S, Widmann CN, Urbach H, Scheltens P, Heneka MT. Clinical symptoms and risk factors in cerebral microangiopathy patients. PLoS ONE. 2013;8:e53455–e53455.
Shindo A, Ishikawa H, Ii Y, Niwa A, Tomimoto H. Clinical features and experimental models of cerebral small vessel disease. Front Aging Neurosci. 2020;12:109.
Jellinger K. Pathology and pathogenesis of vascular cognitive impairment—a critical update. Front Aging Neurosci. 2013;5:17.
Blom K, Emmelot-Vonk MH, Koek HL. The influence of vascular risk factors on cognitive decline in patients with dementia: a systematic review. Maturitas. 2013;76:113–7.
de Liyis BG, Halim W, Widyadharma IPE. Potential role of recombinant growth differentiation factor 11 in Alzheimer’s disease treatment. Egypt J Neurol Psychiatry Neurosurg. 2022;58:49.
Kalaria RN. Neuropathological diagnosis of vascular cognitive impairment and vascular dementia with implications for Alzheimer’s disease. Acta Neuropathol. 2016;131:659–85.
Korczyn AD, Vakhapova V, Grinberg LT. Vascular dementia. J Neurol Sci. 2012;322:2–10.
Vinters HV, Zarow C, Borys E, Whitman JD, Tung S, Ellis WG, et al. Review: vascular dementia: clinicopathologic and genetic considerations. Neuropathol Appl Neurobiol. 2018;44:247–66.
Venkat P, Chopp M, Chen J. Models and mechanisms of vascular dementia. Exp Neurol. 2015;272:97–108.
McKay E, Counts SE. Multi-infarct dementia: a historical perspective. Dement Geriatr Cogn Dis Extra. 2017;7:160–71.
Iemolo F, Duro G, Rizzo C, Castiglia L, Hachinski V, Caruso C. Pathophysiology of vascular dementia. Immun Ageing. 2009;6:13.
Kalaria RN. The pathology and pathophysiology of vascular dementia. Neuropharmacology. 2018;134:226–39.
Unger T, Dahlöf B. Compound 21, the first orally active, selective agonist of the angiotensin type 2 receptor (AT2): implications for AT2 receptor research and therapeutic potential. JRAAS J Renin-Angiotensin-Aldosterone Syst. 2010;11:75–7.
McCarthy CA, Vinh A, Miller AA, Hallberg A, Alterman M, Callaway JK, et al. Direct angiotensin AT2 receptor stimulation using a novel AT2 receptor agonist, compound 21, evokes neuroprotection in conscious hypertensive rats. PLoS ONE. 2014. https://doi.org/10.1371/journal.pone.0095762.
Zhao Z, Bao X, Zhang Z, Liu H, Zhang D. Phloroglucinol derivative compound 21 attenuates cuprizone-induced multiple sclerosis mice through promoting remyelination and inhibiting neuroinflammation. Sci China Life Sci. 2020;63:905–14.
Eldahshan W, Sayed MA, Awad ME, Ahmed HA, Gillis E, Althomali W, et al. Stimulation of angiotensin II receptor 2 preserves cognitive function and is associated with an enhanced cerebral vascular density after stroke. Vascul Pharmacol. 2021;141: 106904.
Jing F, Mogi M, Sakata A, Iwanami J, Tsukuda K, Ohshima K, et al. Direct stimulation of angiotensin II type 2 receptor enhances spatial memory. J Cereb blood flow Metab. 2012;32:248–55.
Yasmin R, Shah M, Khan SA, Ali R. Gelatin nanoparticles: a potential candidate for medical applications. Nanotechnol Rev. 2017;6:191–207.
Hathout RM, Metwally AA. Gelatin nanoparticles. Methods Mol Biol. 2000;2019:71–8.
Huang APH, Lai DM, Hsu YH, Tsai HH, Su CY, Hsu S. An anti-inflammatory gelatin hemostatic agent with biodegradable polyurethane nanoparticles for vulnerable brain tissue. Mater Sci Eng C. 2021;121:111799.
Zhao YZ, Li X, Lu CT, Lin M, Chen LJ, Xiang Q, et al. Gelatin nanostructured lipid carriers-mediated intranasal delivery of basic fibroblast growth factor enhances functional recovery in hemiparkinsonian rats. Nanomed Nanotechnol Biol Med. 2014;10:755–64.
Erdő F, Bors LA, Farkas D, Bajza Á, Gizurarson S. Evaluation of intranasal delivery route of drug administration for brain targeting. Brain Res Bull. 2018;143:155–70.
Yong J, Yan L, Wang J, Xiao H, Zeng Q. Effects of compound 21, a non-peptide angiotensin II type 2 receptor agonist, on general anesthesia-induced cerebral injury in neonatal rats. Mol Med Rep. 2018;18:5337–44.
Steckelings UM, Larhed M, Hallberg A, Widdop RE, Jones ES, Wallinder C, et al. Non-peptide AT2-receptor agonists. Curr Opin Pharmacol. 2011;11:187–92.
Mogi M, Horiuchi M. Effect of angiotensin II type2 receptor on stroke, cognitive impairment and neurodegenerative diseases. Geriatr Gerontol Int. 2013;13:13–8.
Bonferoni MC, Rossi S, Sandri G, Ferrari F, Gavini E, Rassu G, et al. Nanoemulsions for “nose-to-brain” drug delivery. Pharmaceutics. 2019;11:1–17.
Laukkanen L. The angiotensin receptor 2 agonist, compound 21, facilitates TRKB activation and reduces the consequences of stress in mice.
Sahoo N, Sahoo RK, Biswas N, Guha A, Kuotsu K. Recent advancement of gelatin nanoparticles in drug and vaccine delivery. Int J Biol Macromol. 2015;81:317–31.
Singh K, Mishra A. Gelatin nanoparticle: preparation, characterization and application in drug delivery. Int J Pharm Sci Res. 2014;5:2149.
Khan SA. Opportunities and challenges in the techniques used for preparation of gelatin nanoparticles. Pak J Pharm Sci; 33.
Coester CJ, Langer K, Von Br H. Gelatin nanoparticles by two step desolvation a new preparation method, surface modifications and cell uptake. J Microencapsul. 2000;17:187–93.
Carvalho JA, Abreu AS, Ferreira VTP, Gonçalves EP, Tedesco AC, Pinto JG, et al. Preparation of gelatin nanoparticles by two step desolvation method for application in photodynamic therapy. J Biomater Sci Polym Ed. 2018;29:1287–301.
Giri TK. 20—Alginate Containing Nanoarchitectonics for Improved Cancer Therapy. In: Holban AM, Grumezescu AM (eds) Nanoarchitectonics for Smart Delivery and Drug Targeting. William Andrew Publishing, pp. 565–588.
Khramtsov P, Burdina O, Lazarev S, Novokshonova A, Bochkova M, Timganova V, et al. Modified desolvation method enables simple one-step synthesis of gelatin nanoparticles from different gelatin types with any bloom values. Pharmaceutics. 2021;13:1537.
Houshyari A, Heydari M, Bagheri M, Nezafati N. Preparation of gelatin nanoparticles by a water-in-oil emulsion method for water-soluble model drug encapsulation. Mater Today Proc. 2018;5:15800–5.
Samaridou E, Alonso MJ. Nose-to-brain peptide delivery—the potential of nanotechnology. Bioorg Med Chem. 2018;26:2888–905.
Sonvico F, Clementino A, Buttini F, Colombo G, Pescina S, Guterres SS, et al. Surface-modified nanocarriers for nose-to-brain delivery: from bioadhesion to targeting. Pharmaceutics. 2018. https://doi.org/10.3390/pharmaceutics10010034.
Xu J, Tao J, Wang J. Design and application in delivery system of intranasal antidepressants. Front Bioeng Biotechnol. 2020. https://doi.org/10.3389/fbioe.2020.626882.
Islam SU, Shehzad A, Ahmed MB, Lee YS. Intranasal delivery of nanoformulations: a potential way of treatment for neurological disorders. Molecules. 2020;25:1929.
Joachim E, Barakat R, Lew B, Kim KK, Ko C, Choi H. Single intranasal administration of 17β-estradiol loaded gelatin nanoparticles confers neuroprotection in the post-ischemic brain. Nanomed Nanotechnol Biol Med. 2020;29: 102246.
Wong LR, Ho PC. Role of serum albumin as a nanoparticulate carrier for nose-to-brain delivery of R-flurbiprofen: implications for the treatment of Alzheimer’s disease. J Pharm Pharmacol. 2018;70:59–69.
Ahmed HA, Ishrat T, Pillai B, Fouda AY, Sayed MA, Eldahshan W, et al. RAS modulation prevents progressive cognitive impairment after experimental stroke: a randomized, blinded preclinical trial. J Neuroinflammation. 2018;15:229.
Alhusban A, Fouda AY, Pillai B, Ishrat T, Soliman S, Fagan SC. Compound 21 is pro-angiogenic in the brain and results in sustained recovery after ischemic stroke. J Hypertens; 33, https://journals.lww.com/jhypertension/Fulltext/2015/01000/Compound_21_is_pro_angiogenic_in_the_brain_and.21.aspx (2015).
Jackson L, Dong G, Althomali W, Sayed MA, Eldahshan W, Baban B, et al. Delayed administration of angiotensin II type 2 receptor (AT2R) agonist compound 21 prevents the development of post-stroke cognitive impairment in diabetes through the modulation of microglia polarization. Transl Stroke Res. 2020;11:762–75.
Ahmed HA, Ishrat T, Pillai B, Bunting KM, Patel A, Vazdarjanova A, et al. Role of angiotensin system modulation on progression of cognitive impairment and brain MRI changes in aged hypertensive animals—a randomized double- blind pre-clinical study. Behav Brain Res. 2018;346:29–40.
Gallo-Payet N, Guimond M-O, Bilodeau L, Wallinder C, Alterman M, Hallberg A. Angiotensin II, a neuropeptide at the frontier between endocrinology and neuroscience: is there a link between the angiotensin II type 2 receptor and Alzheimer’s disease? Front Endocrinol (Lausanne). 2011;2:17.
Mogi M, Jun I, Horiuchi M. Roles of brain angiotensin II in cognitive function and dementia. Int J Hypertens. 2012;2012: 169649.
Guimond M-O, Gallo-Payet N. The angiotensin II type 2 receptor in brain functions: an update. Int J Hypertens. 2012;2012: 351758.
Steckelings UM, Paulis L, Namsolleck P, Unger T. AT2 receptor agonists: hypertension and beyond. Curr Opin Nephrol Hypertens. 2012;21:142–6.
Ahmed H, Ishrat T. The brain AT2R—a potential target for therapy in Alzheimer’s disease and vascular cognitive impairment: a comprehensive review of clinical and experimental therapeutics. Mol Neurobiol. 2020. https://doi.org/10.1007/s12035-020-01964-9.
Jackson L, Eldahshan W, Fagan SC, Ergul A. Within the brain: the renin angiotensin system. Int J Mol Sci. 2018. https://doi.org/10.3390/ijms19030876.
Kaschina E, Namsolleck P, Unger T. AT2 receptors in cardiovascular and renal diseases. Pharmacol Res. 2017;125:39–47.
Padia SH, Carey RM. AT2 receptors: beneficial counter-regulatory role in cardiovascular and renal function. Pflugers Arch. 2013;465:99–110.
Shabir O, Berwick J, Francis SE. Neurovascular dysfunction in vascular dementia, Alzheimer’s and atherosclerosis. BMC Neurosci. 2018;19:62.
Goel R, Bhat SA, Hanif K, Nath C, Shukla R. Angiotensin II receptor blockers attenuate lipopolysaccharide-induced memory impairment by modulation of NF-κB-mediated BDNF/CREB expression and apoptosis in spontaneously hypertensive rats. Mol Neurobiol. 2018;55:1725–39.
Sampson AK, Irvine JC, Shihata WA, Dragoljevic D, Lumsden N, Huet O, et al. Compound 21, a selective agonist of angiotensin AT2 receptors, prevents endothelial inflammation and leukocyte adhesion in vitro and in vivo. Br J Pharmacol. 2016;173:729–40.
Min L-J, Mogi M, Iwanami J, Jing F, Tsukuda K, Ohshima K, et al. Angiotensin II type 2 receptor-interacting protein prevents vascular senescence. J Am Soc Hypertens. 2012;6:179–84.
Bai Z, Wei M, Li Z, Xiao W. Drosophila Uev1a is dually required for Ben-dependent DNA-damage response and fly mobility. Cell Signal. 2020;74: 109719.
Hodge CD, Spyracopoulos L, Glover JNM. Ubc13: the Lys63 ubiquitin chain building machine. Oncotarget. 2016;7:64471–504.
Ismael S, Ishrat T. Compound 21, a direct AT2R agonist, induces IL-10 and inhibits inflammation in mice following traumatic brain injury. NeuroMolecular Med. 2021. https://doi.org/10.1007/s12017-021-08687-7.
Ahmed HA, Ismael S, Salman M, Devlin P, McDonald MP, Liao F-F, et al. Direct AT2R stimulation slows post-stroke cognitive decline in the 5XFAD Alzheimer’s disease mice. Mol Neurobiol. 2022;59:4124–40.
Eldahshan W, Ishrat T, Pillai B, Sayed MA, Alwhaibi A, Fouda AY, et al. Angiotensin II type 2 receptor stimulation with compound 21 improves neurological function after stroke in female rats: a pilot study. Am J Physiol Circ Physiol. 2019;316:H1192–201.
Jackson-Cowan L, Eldahshan W, Dumanli S, Dong G, Jamil S, Abdul Y, et al. Delayed administration of angiotensin receptor (AT2R) agonist C21 improves survival and preserves sensorimotor outcomes in female diabetic rats post-stroke through modulation of microglial activation. Int J Mol Sci. 2021;22:1356.
Joseph JP, Mecca AP, Regenhardt RW, Bennion DM, Rodríguez V, Desland F, et al. The angiotensin type 2 receptor agonist Compound 21 elicits cerebroprotection in endothelin-1 induced ischemic stroke. Neuropharmacology. 2014;81:134–41.
Acknowledgements
We thank every party involved in the making of this manuscript. The final text has been read by all of the writers and have all given their consent publication. All figures included are original.
Funding
Not applicable.
Author information
Authors and Affiliations
Contributions
The initial concept for this literature review was hatched by BGL. The text was written by BGL, JCS and PMIK with guidance from SL and IPEW. BGL, JCS, PMIK, SL and IPEW completed, copyedited and revised the manuscript. All authors assisted in reviewing, composing the manuscript, creating the figures and reviewing the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
All authors have given their consent for publication.
Competing interests
There is no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
de Liyis, B.G., Sutedja, J.C., Kesuma, P.M.I. et al. A review of literature on Compound 21-loaded gelatin nanoparticle: a promising nose-to-brain therapy for multi-infarct dementia. Egypt J Neurol Psychiatry Neurosurg 59, 13 (2023). https://doi.org/10.1186/s41983-023-00621-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-023-00621-x