Abstract
Background
Most of the patients with Parkinson’s disease (PD) suffer from non-motor symptoms (NMS). Despite their marked effect on patients’ quality of life, NMS remain under-estimated by physicians, patients and caregivers. The aim of this study was to suggest a battery to screen for the presence of NMS in PD patients in the setting of an outpatient clinic and to assess the NMS of PD in Egyptian patients and factors affecting them.
Results
This study was conducted on 50 patients with PD, 35 males (70%) and 15 females (30%) whom their age ranged from 36 to 80 years with a mean of 62.88 ± 8.74 years. All patients were assessed using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS), Apathy Scale (AS), Montreal Cognitive Assessment (MOCA), Hamilton Depression Rating Scale (HDRS) and Parkinson’s Disease Sleep Scale (PDSS). The mean age at disease onset was 59.10 ± 9.34 years and the mean disease duration was 3.76 ± 3.16 years. 98% of patients (49 out of 50) had at least one non-motor symptom. The mean MDS-UPDRS scores were 15.74 ± 7.93 for part I, 17.94 ± 11.61 for part II and 42.32 ± 22.74 for part III. The mean score for AS was 9.90 ± 10.66 with 70% of patients considered apathetic. The mean MOCA score was 21.12 ± 4.73 with 38 patients (76%) found to be cognitively impaired. The mean HDRS score was 12.26 ± 8.52 with 34 patients (68%) found to be depressed. The mean PDSS score was 92.22 ± 32.53 with sleep disturbances found in 38 patients (76%). A statistically significant negative correlation was found between the HDRS and PDSS scores (P value < 0.001). Age of patients, age at disease onset and disease duration were not correlated to MDS-UPDRS, AS, MOCA, HDRS and PDSS scores.
Conclusions
Most of the patients with PD were found to suffer from NMS including apathy, cognitive impairment, depression and sleep disturbances. Physicians need to screen their PD patients for NMS on regular basis using the appropriate tools. Self-administered questionnaires could function as reliable screening tools for NMS in PD patients.
Similar content being viewed by others
Background
Parkinson’s disease (PD) is believed to be the second most common neurodegenerative disease [1]. In 2016, the global burden of disease (GBD) study estimated the global prevalence of PD to be 6.1 million (95% uncertainty interval [UI] 5.0–7.3) with PD causing 3.2 million (95% UI 2.6–4.0) disability-adjusted life years (DALYs). For Egypt, the GBD study estimated a prevalence of 48,694 (39,464–59,862) and 24,460 (18,678–31,133) DALYs in 2016 [2].
At least one-third of PD patients [3] and in other reports 100% of PD patients [4, 5] suffer from non-motor symptoms (NMS) which may precede the onset of motor symptoms and the diagnosis of PD [6,7,8]. NMS of PD include autonomic, cognitive, neuropsychiatric, sensory and sleep dysfunction [9]. NMS such as constipation, depression, olfactory dysfunction and rapid eyeball movement sleep behavior disorder (RBD) can present in the prodromal phase of PD [10].
Pathology of NMS of PD is believed to involve brain areas other than the dopaminergic nigrostriatal system, such as dorsal vagal nucleus, hypothalamus, limbic cortex, locus coeruleus, neocortex, olfactory tubercle and raphe nuclei of the brainstem [11, 12]. Pathology of PD also involves the peripheral autonomic nervous system including the myenteric plexus and sympathetic ganglia [12]. Also, medications used to treat motor symptoms of PD can induce NMS such as hallucinations, orthostatic hypotension and somnolence [13].
Focusing only on motor symptoms and ignoring NMS may not reflect the patient’s experience of living with PD [14]. Non-motor symptoms negatively impact the quality of life of PD patients and add to their disease burden and disability [15].
The aim of this study was to suggest a battery to screen for the presence of NMS in PD patients in the setting of an outpatient clinic and to assess the NMS of PD in Egyptian patients and factors affecting them.
Methods
The study recruited 50 patients with idiopathic PD recruited from the movement disorders clinic of Cairo University Hospital from March 2021 to January 2022. The study population included 35 males (70%) and 15 females (30%) whom their age ranged from 36 to 80 years with a mean value of 62.88 ± 8.74 years, 2 of patients were below 50 years. Patients were diagnosed with PD according to the UK PDS Brain Bank Criteria for the diagnosis of PD [16]. The study protocol was revised and approved by the institutional review board of faculty of medicine—Cairo University (MS-91-2021) and a written informed consent was obtained from all recruited patients.
All patients were assessed for stage and severity of PD using the Movement Disorder Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) Arabic version [17]. The MDS-UPDRS is composed of four parts: part 1, non-motor experiences of daily living; part 2, motor experiences of daily living; part 3, motor examination; part 4, motor complications.
Non-motor symptoms were assessed in all patients using the following set of scales:
Apathy Scale (AS) (Arabic version) [18]: AS is a 14-question inventory that screens for apathy and assesses its severity over the 4 weeks prior to assessment. Each question is scored from 0 to 3, with the total score (range 0–42) calculated by summing the scores of all questions. Questions 1 through 8 are scored on a scale from 3 (not at all) to 0 (a lot), whereas questions 9 through 14 are scored on a scale from 0 (not at all) to 3 (a lot). A score of 14 or higher indicates that a patient is apathetic.
Montreal Cognitive Assessment (MOCA) (type A for educated patients) and (type B for non-educated patients) (Arabic version) [19]: MOCA assesses attention, concentration, calculations, conceptual thinking, executive functions, memory, language, orientation and visuoconstructional skills. The maximum possible score is 30 points. According to MOCA developers, patients scoring between 18 and 25 were considered to be mildly impaired (www.mocatest.org). Diagnosis of PD dementia (PDD) was achieved with a score below 18 [20].
Hamilton Depression Rating Scale (HDRS) (Arabic version) [21]: the 17 questions version of HDRS was used in this study. A score of 0–7 is normal, a score of 8–16 suggests mild depression, a score of 7–23 suggests moderate depression and scores over 24 indicate severe depression.
Parkinson’s Disease Sleep Scale (PDSS) (Arabic version) [22]: The PDSS includes 15 questions addressing the following domains: overall quality of night’s sleep, sleep onset and maintenance insomnia, nocturnal restlessness, nocturnal psychosis, nocturia, nocturnal motor symptoms, sleep refreshment and daytime dozing. The response is given on a scale from 0 to 10, where 0 indicates that a symptom is severe and always experienced, while 10 means being free of symptoms. The total score ranges from 0 (most severe) to 150 (free of symptoms). When a subject obtains an overall score below 82 or a score under 5 on any sub-item of the PDSS, he/she is considered to have sleep disorder.
These four scales (AS, MOCA, HDRS and PDSS) together present the suggested battery of self-administered questionnaires proposed to be used for screening for NMS in PD patients.
Data were coded and entered using the statistical package for the Social Sciences (SPSS) version 26 (IBM Corp., Armonk, NY, USA). Data were summarized using mean, standard deviation, median, minimum and maximum in quantitative data and using frequency (count) and relative frequency (percentage) for categorical data. Comparisons between quantitative variables were done using the non-parametric Mann–Whitney test. For comparing categorical data, Chi-square (χ2) test was performed. Exact test was used instead when the expected frequency is less than 5. Correlations between quantitative variables were done using Spearman correlation coefficient. P-values less than 0.05 were considered as statistically significant.
Results
Seventy percent (n = 35) of patients included in this study were males while 30% (n = 15) were females. Table 1 shows the demographic and clinical characteristics of study population.
Ninety-eight percent of patients in our cohort (49 out of 50) had at least one of the NMS they were screened for (apathy, cognitive impairment, depression and sleep disturbances). Only one patient of our cohort scored zero in first part of MDS-UPDRS assessing the non-motor experiences of daily living. Table 2 shows the mean MDS-UPDRS scores and severity of PD as assessed by MDS-UPDRS. Since none of our cohort suffered from dyskinesias, part 4 of MDS-UPDRS was not applied.
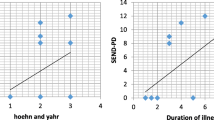
Scores of 1st part of MDS-UPDRS assessing the non-motor experiences of daily living were significantly positively correlated to scores of HDRS and significantly negatively correlated to scores of PDSS (P value < 0.001 for both) but not to scores of AS or MOCA. Table 3 shows a comparison between affected and non-affected patients in each scale (HDRS, AS, PDSS and MOCA) as regards severity according to Part 1 of MDS-UPDRS. Scores of 1st part of MDS-UPDRS were also significantly positively correlated to the scores of the 2nd and 3rd parts of the MDS-UPRDS assessing the motor experiences of daily living and motor examination as shown in Table 4.
The mean score for AS was 9.90 ± 10.66 with 70% of patients scoring 14 or higher and were considered apathetic. There was a statistically significant positive correlation between AS scores and the scores of the 2nd part of MDS-UPRDS assessing the motor experiences of daily living (P value 0.035). Such correlation was not found with the 1st and 3rd parts of the MDS-UPRDS.
Thirty-three patients (66%) performed the MOCA type A test, while 17 patients (34%) were illiterate and performed the MOCA type B test. The mean MOCA score was 21.12 ± 4.73. Thirty-eight patients (76%) were found to be cognitively impaired, 25 patients (65.8%) of them scored 18–25 and were found to have mild cognitive impairment and 9 patients (23.7%) scored below 18 and were considered demented and had moderate-to-severe affection in activities of daily living. There was a statistically significant negative correlation between MOCA scores and the scores of the 3rd part of MDS-UPRDS assessing the motor examination (P value 0.022) and the total MDS-UPDRS scores (P value 0.010). No correlation was found with the scores of the 1st and 2nd parts of MDS-UPDRS.
The mean HDRS score was 12.26 ± 8.52. Thirty-four patients (68%) were found to be depressed [19 patients (56%), mild depression; 9 patients (26%), moderate depression; 6 patients (18%), severe depression]. There was a statistically significant positive correlation between the HDRS scores and the scores of all 3 parts of MDS-UPRDS (P value < 0.001, < 0.001 and 0.024, respectively).
The mean PDSS score was 92.22 ± 32.53. Sleep disturbances was found in 38 patients (76%) with nocturia being the most frequently reported sleep disturbance in 34 patients (68%) followed by nocturnal motor symptoms (nocturnal muscle cramps and early morning dystonia) in 29 patients (58%). There was a statistically significant negative correlation between the PDSS scores the scores of all 3 parts of MDS-UPRDS (P value < 0.001, < 0.001 and 0.008, respectively).
When correlating the scores of the AS, MOCA, HDRS and PDSS to each other, only a statistically significant negative correlation was found between the HDRS and PDSS scores (P value < 0.001). Age of patients, age at disease onset and disease duration were not correlated to MDS-UPDRS, AS, MOCA, HDRS and PDSS scores.
Discussion
Non-motor symptoms of PD add substantially to morbidity, dependency, hospitalization and increases in costs of medical care [23]. In this study, we assessed the NMS of PD in Egyptian patients and factors affecting them and we suggested a battery to screen for the presence of NMS in PD in the setting of an outpatient clinic. Our battery consisted of the first part of MDS-UPDRS for screening of all NMS, AS for apathy assessment, MOCA scale for assessment of cognitive function, HDRS for assessment of depression and PDSS for assessment of sleep dysfunction. All scales are self-administrated which offers an opportunity to assess NMS while the patients are at the waiting area in the outpatient clinic before meeting their treating physicians.
Ninety-eight percent of patients in this cohort (49 out of 50) had at least one non-motor symptom. Only one patient of this cohort scored zero in first part of MDS-UPDRS assessing the non-motor experiences of daily living. These findings are similar to several studies which showed a prevalence of NMS in PD ranging from 98 to 100% [5, 24,25,26,27,28,29].
Total scores of 1st part of MDS-UPDRS were correlated to HDRS and PDSS scores but not to MOCA scores. Marius and colleagues stated that a bidirectional temporal relation exists between different non-motor symptoms (cognitive impairment, depression, hallucinations and daytime sleepiness) [30].
Age of patients, age at disease onset and disease duration were not correlated to any of the scales used in our study to assess NMS. Such correlations were established in other studies [31,32,33]. A larger sample size may be needed to examine the correlation between NMS and these potential risk factors. Another point that should be considered as regards the correlation to age at disease onset and disease duration is that patients with low educational level may not catch the accurate time when their symptoms started. Some patients and caregivers with low socio-economic backgrounds and low educational levels may consider bradykinesia and tremors as part of the normal aging process and do not seek medical advice as soon as PD symptoms start [34].
Thirty percent of patients in this cohort were found apathetic when assessed with the AS. In a recent systematic review, Mele and colleagues found that 29.1% of PD patients were apathetic [35]. Other studies reported different prevalence rates of apathy ranging from 12.7% to 51% [36, 37]. AS scores were not correlated with MDS-UPDRS, MOCA, HDRS and PDSS scores in our study. Yet only 15 patients of this cohort were found to have apathy, a bigger sample size may be needed to study these relations.
Seventy-six percent of PD patients in this cohort were found to be cognitively impaired when assessed using the MOCA scale. Two previous Egyptian studies assessed cognitive impairment in PD patients. One study used the Non-Motor Symptoms Scale (NMSS) and found that 87% of patients were cognitively impaired with a mean duration of disease of 5 years [38]. The other study used the MOCA scale and detected cognitive impairment in 25% of patients, but this study included only newly diagnosed patients [34].
Reaching a definite diagnosis of dementia in PD patients can be difficult due to the interplay between apathy, depression, hallucinations, motor symptoms and sleep insufficiency in these patients [20]. In this cohort, a statistically significant negative correlation between MOCA scores and the scores of the 3rd part of MDS-UPRDS assessing the motor examination was found. This relation between motor symptoms and cognition is well established in PD and was replicated in many studies [39,40,41,42,43,44].
In this study, 68% of PD patients assessed with HDRS suffered from depression. Shalash and colleagues in a cohort of 97 Egyptian PD patients found that 76.7% of patients were depressed using Beck Depression Inventory [38]. Ragab and colleagues in their cohort found a depression prevalence of 47.5% in newly diagnosed patients [34], while Khedr and colleagues reported a depression prevalence of 31.25% in their cohort from upper Egypt [45]. Several studies outside Egypt found a prevalence of depression among PD patients ranging from 7 to 76% [46,47,48]. Such disparity may be explained by variations in study populations, diagnostic tools used, and types of depressive disorders included in different studies; major depressive disorder, minor depression or dysthymia.
In this study, there was a strong positive correlation between the severity of depression according to HADRS and other NMS according to MDS-UPDRS part I. An Indian study conducted on 126 PD patients found a significant association between severity of PD and depression [49]. Also, Gallagher and colleagues in a cohort of 96 PD patients found a significant relationship between the MDS-UPDRS part I scores and severity of depression [50].
In this study, depression as assessed by the HDRS was not correlated to age of patients, age of onset of PD, duration of disease. This could be explained by the fact that dopaminergic dysfunction plays the main role in depression as proven by previous biomarker and neuroimaging studies [51].
Sleep disturbances were reported in 76% of our study cohort as assessed by PDSS. Prevalence of sleep disturbances in PD ranged from 40 to 98% in most of global and Egyptian studies [5, 34, 38, 45]. These wide variations reflect differences in patients’ populations and methods used to assess sleep; whether polysomnography or only questionnaires. Nocturia was the most frequent form of sleep disorder in our patients (68%). This was similar to several previous studies [52, 53]. Nocturia may be attributed to incomplete bladder emptying which reflects autonomic dysfunction in PD patients, also nocturia may occur as the dose of L-dopa wears off [52].
We found a strong negative correlation between PDSS scores and the severity of NMS of PD as assessed by the MDS-UPDRS part I. Also, PDSS scores were correlated to severity of PD motor symptoms as assessed by MDS-UPDRS parts II and III. Similar results were found in a multi-center cross-sectional study which included 436 patients in Japan [54]. Sleep disturbances can occur at any stage of PD, but the severity and frequency increase as the disease advances. This could be due to worsening nocturnal motor complications such as akinesia, cramps and dystonia as the pathology of PD progresses [52].
This study shed light on the importance of increasing awareness about NMS of PD among physicians, patients and caregivers. This study also confirmed the feasibility of using self-administrated questionnaires to screen for NMS in PD patients in outpatient clinics. However, patients should be referred to more detailed assessments and investigations according to the initial screening to reach a final diagnosis.
This study was limited by the relatively small sample size which affected the results and limited further sub-grouping of patients (for example, treated versus treatment-naïve patients). Also, the presence of patients with relatively extreme age and disease duration affected the overall results especially with the small sample size. Further research needs to be conducted with larger sample size covering different geographical areas in different centers across Egypt.
Conclusions
NMS pose a real burden on PD patients and markedly affect their life quality. Most of the patients with PD were found to suffer from NMS including apathy, cognitive impairment, depression and sleep disturbances. Physicians need to screen their PD patients for NMS on a regular basis using the appropriate tools. Self-administered questionnaires could function as reliable screening tools for NMS in PD patients.
Availability of data and materials
The data sets generated and/or analyzed during the current study are not publicly available due to the current Egyptian clinical research legislation, but are available from the corresponding author on reasonable request and after institutional approval.
Abbreviations
- PD:
-
Parkinson’s disease
- GBD:
-
Global burden of disease
- UI:
-
Uncertainty interval
- DALYs:
-
Disability-adjusted life years
- NMS:
-
Non-motor symptoms
- RBD:
-
Rapid eyeball movement sleep behavior disorder
- UK PDS:
-
United Kingdom Parkinson’s Disease Society
- MDS-UPDRS:
-
Movement Disorder Society Unified Parkinson’s Disease Rating Scale
- AS:
-
Apathy Scale
- MOCA:
-
Montreal Cognitive Assessment
- PDD:
-
Parkinson’s disease dementia
- HDRS:
-
Hamilton Depression Rating Scale
- PDSS:
-
Parkinson’s Disease Sleep Scale
- SPSS:
-
Statistical Package for Social Sciences
- NMSS:
-
Non-Motor Symptoms Scale
References
Tysnes OB, Storstein A. Epidemiology of Parkinson’s disease. J Neural Transm (Vienna). 2017;124(8):901–5. https://doi.org/10.1007/s00702-017-1686-y.
GBD 2016 Parkinson’s Disease Collaborators. Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2018;17(11):939–953. https://doi.org/10.1016/S1474-4422(18)30295-3.
Shulman LM, Taback RL, Bean J, Weiner WJ. Comorbidity of the nonmotor symptoms of Parkinson’s disease. Mov Disord. 2001;16(3):507–10. https://doi.org/10.1002/mds.1099.
Gulunay A, Cakmakli GY, Yon MI, Ulusoy EK, Karakoc M. Frequency of non-motor symptoms and their impact on the quality of life in patients with Parkinson’s disease: a prospective descriptive case series. Psychogeriatrics. 2020;20(2):206–11. https://doi.org/10.1111/psyg.12489.
Chaudhuri JR, Mridula KR, Bandaru VCSS. Prevalence of non-motor symptoms in Parkinson’s disease: a study from South India. Turk J Neurol. 2021;27(1):52–7. https://doi.org/10.4274/tnd.2021.52993.
Goetz CG, Lutge W, Tanner CM. Autonomic dysfunction in Parkinson’s disease. Neurology. 1986;36:73–5.
Magalhaes M, Wenning GK, Daniel SE, Quinn NP. Autonomic dysfunction in pathologically confirmed multiple system atrophy and idiopathic Parkinson’s disease—a retrospective comparison. Acta Neurol Scand. 1995;91:98–102.
Santamaria J, Tolosa E, Valles A. Parkinson’s disease with depression: a possible subgroup of idiopathic parkinsonism. Neurology. 1986;36:1130–3.
Chaudhuri KR, Healy DG, Schapira AH, National Institute for Clinical Excellence. Non-motor symptoms of Parkinson’s disease: diagnosis and management. Lancet Neurol. 2006;5:235–45.
Postuma RB, Aarsland D, Barone P, Burn DJ, Hawkes CH, Oertel W, et al. Identifying prodromal Parkinson’s disease: pre-motor disorders in Parkinson’s disease. Mov Disord. 2012;27:617–26.
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211.
Forno LS. The neuropathology of Parkinson’s Disease. In: Hefti F, Weiner WJ, editors. Progress in Parkinson research. Boston: Springer; 1988. https://doi.org/10.1007/978-1-4613-0759-4_2.
Poewe W. Non-motor symptoms in Parkinson’s disease. Eur J Neurol. 2008;15(Suppl 1):14–20. https://doi.org/10.1111/j.1468-1331.2008.02056.x.
Carroll V, Rossiter R, Blanchard D. Non-motor symptoms of Parkinson’s disease. Aust J Gen Pract. 2021;50(11):812–7. https://doi.org/10.31128/AJGP-07-21-6093.
Stiasny-Kolster K, Magerl W, Oertel WH, Moller JC, Treede RD. Static mechanical hyperalgesia without dynamic tactile allodynia in patients with restless legs syndrome. Brain. 2004;127:773–82.
Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases. JNNP. 1992;55:181–4.
Khalil H, Aldaajani ZF, Aldughmi M, Al-Sharman A, Mohammad T, Mehanna R, et al. Validation of the arabic version of the movement disorder society-unified Parkinson’s disease rating scale. Mov Disord. 2022;37(4):826–41. https://doi.org/10.1002/mds.28905.
Starkstein SE, Mayberg HS, Preziosi TJ, Andrezejewski P, Leiguarda R, Robinson RG. Reliability, validity, and clinical correlates of apathy in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 1992;4(2):134–9. https://doi.org/10.1176/jnp.4.2.134.
Saleh AA, Alkholy RSAEHA, Khalaf OO, Sabry NA, Amer H, El-Jaafary S, et al. Validation of Montreal Cognitive Assessment-Basic in a sample of elderly Egyptians with neurocognitive disorders. Aging Ment Health. 2019;23(5):551–7. https://doi.org/10.1080/13607863.2018.1428936.
Dubois B, Burn D, Goetz C, Aarsland D, Brown RG, Broe GA, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22(16):2314–24. https://doi.org/10.1002/mds.21844.
Sharp R. The Hamilton rating scale for depression. Occup Med (Lond). 2015;65(4):340. https://doi.org/10.1093/occmed/kqv043.
Chaudhuri KR, Pal S, DiMarco A, Whately-Smith C, Bridgman K, Mathew R, et al. The Parkinson’s disease sleep scale: a new instrument for assessing sleep and nocturnal disability in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2002;73(6):629–35. https://doi.org/10.1136/jnnp.73.6.629.
Lundqvist C, Beiske AG, Reiertsen O, Kristiansen IS. Real life cost and quality of life associated with continuous intraduodenal levodopa infusion compared with oral treatment in Parkinson patients. J Neurol. 2014;261(12):2438–45. https://doi.org/10.1007/s00415-014-7515-4.
Barone P, Antonini A, Colosimo C, Marconi R, Morgante L, Avarello TP, et al. The PRIAMO study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson’s disease. Mov Disord. 2009;24(11):1641–9. https://doi.org/10.1002/mds.22643.
Li H, Zhang M, Chen L, Zhang J, Pei Z, Hu A, et al. Nonmotor symptoms are independently associated with impaired health-related quality of life in Chinese patients with Parkinson’s disease. Mov Disord. 2010;25(16):2740–6. https://doi.org/10.1002/mds.23368.
Zhang H, Gu Z, An J, Wang C, Chan P. Non-motor symptoms in treated and untreated Chinese patients with early Parkinson’s disease. Tohoku J Exp Med. 2014;232(2):129–36. https://doi.org/10.1620/tjem.232.129.
Rukmini Mridula K, Borgohain R, Jabeen SA, Padmaja G, Bandaru VS, Ankathi P, et al. Comparison of frequencies of non motor symptoms in Indian Parkinson’s disease patients on medical management versus deep brain stimulation: a case-control study. Iran J Neurol. 2015;14(2):86–93.
Liu WM, Lin RJ, Yu RL, Tai CH, Lin CH, Wu RM. The impact of nonmotor symptoms on quality of life in patients with Parkinson’s disease in Taiwan. Neuropsychiatr Dis Treat. 2015;11(11):2865–73. https://doi.org/10.2147/NDT.S88968.
Ravan A, Ahmad FM, Chabria S, Gadhari M, Sankhla CS. Non-motor symptoms in an Indian cohort of Parkinson’s disease patients and correlation of progression of non-motor symptoms with motor worsening. Neurol India. 2015;63(2):166–74. https://doi.org/10.4103/0028-3886.156276.
Marinus J, Zhu K, Marras C, Aarsland D, van Hilten JJ. Risk factors for non-motor symptoms in Parkinson’s disease. Lancet Neurol. 2018;17(6):559–68. https://doi.org/10.1016/S1474-4422(18)30127-3.
Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci. 2017;18(8):509. https://doi.org/10.1038/nrn.2017.91.
Kitani-Morii F, Kasai T, Horiguchi G, Teramukai S, Ohmichi T, Shinomoto M, et al. Risk factors for neuropsychiatric symptoms in patients with Parkinson’s disease during COVID-19 pandemic in Japan. PLoS ONE. 2021;16(1): e0245864. https://doi.org/10.1371/journal.pone.0245864.
Rizos A, Martinez-Martin P, Odin P, Antonini A, Kessel B, Kozul TK, et al. Characterizing motor and non-motor aspects of early-morning off periods in Parkinson’s disease: an international multicenter study. Parkinsonism Relat Disord. 2014;20(11):1231–5. https://doi.org/10.1016/j.parkreldis.2014.09.013.
Ragab OA, Elheneedy YA, Bahnasy WS. Non-motor symptoms in newly diagnosed Parkinson’s disease patients. Egypt J Neurol Psychiatry Neurosurg. 2019;55:24. https://doi.org/10.1186/s41983-019-0070-2.
Mele B, Merrikh D, Ismail Z, Goodarzi Z. Detecting apathy in individuals with Parkinson’s disease: a systematic review. J Parkinsons Dis. 2019;9(4):653–64. https://doi.org/10.3233/JPD-191619 (PMID: 31424418).
Robert G, Le Jeune F, Lozachmeur C, Drapier S, Dondaine T, Péron J, et al. Apathy in patients with Parkinson disease without dementia or depression: a PET study. Neurology. 2012;79(11):1155–60. https://doi.org/10.1212/WNL.0b013e3182698c75.
Sousa M, Moreira F, Jesus-Ribeiro J, Marques I, Cunha F, Canário N, et al. Apathy profile in Parkinson’s and Huntington’s disease: a comparative cross-sectional study. Eur Neurol. 2018;79(1–2):13–20. https://doi.org/10.1159/000481981.
Shalash AS, Hamid E, Elrassas HH, Bedair AS, Abushouk AI, Khamis M, et al. Non-motor symptoms as predictors of quality of life in Egyptian patients with Parkinson’s disease: a cross-sectional study using a culturally adapted 39-item Parkinson’s disease questionnaire. Front Neurol. 2018;24(9):357. https://doi.org/10.3389/fneur.2018.00357.
Wang YQ, Tang BS, Yan XX, Chen ZH, Xu Q, Liu ZH, et al. A neurophysiological profile in Parkinson’s disease with mild cognitive impairment and dementia in China. J Clin Neurosci. 2015;22(6):981–5. https://doi.org/10.1016/j.jocn.2014.11.030.
Jones JD, Mangal P, Lafo J, Okun MS, Bowers D. Mood differences among Parkinson’s disease patients with mild cognitive impairment. J Neuropsychiatry Clin Neurosci. 2016;28(3):211–6. https://doi.org/10.1176/appi.neuropsych.15090221.
Hong JY, Lee Y, Sunwoo MK, Sohn YH, Lee PH. Subjective cognitive complaints and objective cognitive impairment in Parkinson’s disease. J Clin Neurol. 2018;14(1):16–21. https://doi.org/10.3988/jcn.2018.14.1.16.
Orfei MD, Assogna F, Pellicano C, Pontieri FE, Caltagirone C, Pierantozzi M, et al. Anosognosia for cognitive and behavioral symptoms in Parkinson’s disease with mild dementia and mild cognitive impairment: frequency and neuropsychological/neuropsychiatric correlates. Parkinsonism Relat Disord. 2018;54:62–7. https://doi.org/10.1016/j.parkreldis.2018.04.015.
Monastero R, Cicero CE, Baschi R, Davì M, Luca A, Restivo V, et al. Mild cognitive impairment in Parkinson’s disease: the Parkinson’s disease cognitive study (PACOS). J Neurol. 2018;265(5):1050–8. https://doi.org/10.1007/s00415-018-8800-4.
Baiano C, Barone P, Trojano L, Santangelo G. Prevalence and clinical aspects of mild cognitive impairment in Parkinson’s disease: a meta-analysis. Mov Disord. 2020;35(1):45–54. https://doi.org/10.1002/mds.27902.
Khedr EM, Abdelrahman AA, Elserogy Y. Depression and anxiety among patients with Parkinson’s disease: frequency, risk factors, and impact on quality of life. Egypt J Neurol Psychiatry Neurosurg. 2020;56:116. https://doi.org/10.1186/s41983-020-00253-5.
Veazey C, Aki SO, Cook KF, Lai EC, Kunik ME. Prevalence and treatment of depression in Parkinson’s disease. J Neuropsychiatry Clin Neurosci. 2005;17(3):310–23. https://doi.org/10.1176/jnp.17.3.310.
Reijnders JS, Ehrt U, Weber WE, Aarsland D, Leentjens AF. A systematic review of prevalence studies of depression in Parkinson’s disease. Mov Disord. 2008;23(2):183–9. https://doi.org/10.1002/mds.21803.
Hermanowicz N, Jones SA, Hauser RA. Impact of non-motor symptoms in Parkinson’s disease: a PMDAlliance survey. Neuropsychiatr Dis Treat. 2019;5(15):2205–12. https://doi.org/10.2147/NDT.S213917.
Rai NK, Goyal V, Kumar N, Shukla G, Srivastava AK, Singh S, et al. Neuropsychiatric co-morbidities in non-demented Parkinson’s disease. Ann Indian Acad Neurol. 2015;18:33–8.
Gallagher DA, Goetz GC, Stebbins G, Less JA, Schrag A. Validation of MDS-UPDRS part I for nonmotor symptoms in Parkinson’s disease. Wiley Online Library. 2011;1(27):79–83.
Cui SS, Du JJ, Fu R, Lin QY, Huang P, He YC, et al. Prevalence and risk factors for depression and anxiety in Chinese patients with Parkinson disease. BMG Geriatr. 2017;17:1–10.
Kumar S, Bhatia M, Behari M. Sleep disorders in Parkinson’s disease. Mov Disord. 2002;17(4):775–81. https://doi.org/10.1002/mds.10167.
Batla A, Phé V, De Min L, Panicker JN. Nocturia in Parkinson’s disease: why does it occur and how to manage? Mov Disord Clin Pract. 2016;3(5):443–51. https://doi.org/10.1002/mdc3.12374.
Suzuki K, Okuma Y, Uchiyama T, Miyamoto M, Sakakibara R, Shimo Y, et al. Impact of sleep-related symptoms on clinical motor subtypes and disability in Parkinson’s disease: a multicentre cross-sectional study. J Neurol Neurosurg Psychiatry. 2017;88(11):953–9. https://doi.org/10.1136/jnnp-2017-316136.
Acknowledgements
The authors are grateful to all participants for their willingness to participate in this study.
Funding
There is no source of funding for the research.
Author information
Authors and Affiliations
Contributions
SS, RMA, MF, OE and MIH carried out the work. OE and MF designed the protocol. MIH, SS and RMA shared collected scientific data. MIH was responsible for writing the initial draft of the manuscript. MF, MIH and SS did the statistical analysis. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
The authors obtained permission to conduct this study that was approved by the Institutional Review Board (IRB), Faculty of Medicine—Cairo University (MS-91-2021). All participants signed an informed consent. The procedures followed were in accordance with our protocol. We recruited 50 patients from the movement disorders clinic of Cairo university hospital.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shaheen, S., Ali, R.M., Farghaly, M. et al. Screening for non-motor symptoms in Egyptian patients with Parkinson’s disease. Egypt J Neurol Psychiatry Neurosurg 58, 103 (2022). https://doi.org/10.1186/s41983-022-00541-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41983-022-00541-2