Abstract
Background
Species belonging to the genus Trichoderma are considered as one of the most potential biocontrol agents which can be readily collected from soil and found effective against various fungal and bacterial diseases. In rice-growing areas, the major fungal pathogens affecting rice production include Rhizoctonia solani causing sheath blight and Sclerotium oryzae causing stem rot disease of rice. Due to the lack of resistant varieties and detrimental effects of chemicals, biocontrol gives a good opportunity to manage the diseases efficiently in a sustainable manner.
Main body
Trichoderma spp. from native rice rhizosphere soil were examined for their antagonistic efficiency to supress the two soil-borne rice pathogens, viz., R. solani and S. oryzae. Morphological, biochemical and molecular characterisation of the isolates led to the identification of species as T. asperellum. The isolates of Trichoderma spp. were found to be positive to IAA release and phosphate solubilisation and were screened against R. solani and S. oryzae in vitro and in vivo in pots under glass house conditions. Regression analysis indicated a positive correlation between the amount of chlamydospores produced by T. asperellum and their antagonistic potential against the two pathogens. Exposure to external stimuli, viz., light, injury and nitrogen sources in culture media triggered increased conidiation in Trichoderma isolates. Among the four isolates studied, Trichoderma asperellum IIRRCK1 (TAIK-1) was found to be the most effective in improving plant growth in rice and highly antagonistic against R. solani and S. oryzae. Sorghum grain was found to be the most suitable among different organic substrates studied to provide better growth and viability of TAIK-1 and improved the efficiency of the seed treatment and soil application. External stimuli in the form of near UV blue light, mechanical injury to the colonies and nitrogen source added to the culture media help in faster conidiation of Trichoderma.
Conclusion
Strain TAIK-1 showed strong competitive and antagonistic activities against fungal soil-borne pathogens, in addition with promoting healthy growth and development of rice plants. This can be a suitable and safe alternative to chemical management in the rice fields for long-term scenario.
Similar content being viewed by others
Background
About half the world population and more than 50% of India’s population consume rice as their staple diet, and ensuring sustainable rice production in this context is important for the food security of the nation and ensuring food supply to the global population. Introduction of semi-dwarf, early maturing and fertiliser responsive high-yielding rice varieties since early 1970s resulted in an increased incidence of pests and diseases in tropical rice-growing countries like India (Hussain 2015). Sheath blight caused by Rhizoctonia solani and stem rot caused by Sclerotium oryzae are important soil-borne diseases causing a yield loss of about 4–50 and 3–45%, respectively (Nagarajkumar et al. 2004). Agrochemicals used in disease management are effective and give a rapid recovery in the same cropping cycle, but come with a high cost on the environment (Rakesh et al. 2017). Further, the unwise usage and underdose applications of chemicals have the risk of resistance development in the target fungal pathogens (Deising et al. 2008). Development of resistant varieties against these 2 diseases is still a distant dream because of the non-availability of resistant genes in the host due to the saprophytic and opportunistic nature of the pathogens with a wide host base and non-specific pathogenesis that can trigger pathogen-specific resistant reactions by the host (Rosas et al. 2016). Under these circumstances, biological control offers a suitable and potential alternative for the management of these two pathogens.
The use of bioagents to suppress the disease-causing pathogens and their progress in the host is referred to as biological control (Sharma et al. 2013). Microbial bioagents (or antagonists) employ several methods including hyper-parasitism, competition for nutrients and space, antibiosis and induction of systemic resistance in the host plants against the pathogens to dominate and suppress the pathogens (Sharma et al. 2013). The key to successful biocontrol involves (a) a potential bioagent against the target pathogen; (b) proper delivery in a suitable active form in the right target area; (c) creating favourable environment, selective to the bioagent; (d) cultivating varieties that support the bioagent; and (e) periodically monitoring the dynamics of the bioagent and augmenting the bioagent populations as and when required (Gnanamanickam 2009).
Biological control in rice has been deployed with limited success, dominated by the use of the fungi Trichoderma spp. Trichoderma commonly occurs in soil, bark, woods and rhizosphere of plants, as either ectophytic or endophytic inside the plant tissues (Verma et al. 2007). Trichoderma spp. also exhibit plant growth promoting activities, helping the plants to maintain a healthy defence against the invading pathogens. Its unique property of producing phytohormones, fungal cell wall digestive enzymes, antimicrobial secondary metabolites and volatile organic compounds have created more interest among researchers to isolate, identify and use several of their species.
Despite all the advantages, field-level success in the deployment of Trichoderma spp. is still eluding in the case of rice crop, mainly because of the varied growing conditions of rice like flooded, continuously submerged or aerobic dry conditions (Gnanamanickam 2009). These problems can be overcome by developing effective formulations with a long shelf life, use of more resilient strains and involving the farming community to multiply the bioagents in situ at their farm level.
The present study focused on the use of Trichoderma asperellum, its mass multiplication using commonly available organic wastes, and assessment of its efficiency in plant growth promotion and disease suppression against 2 important soil-borne pathogens of rice, viz., R. solani and S. oryzae.
Main text
Materials and methods
Bioagents and pathogens used in the study
Isolates of T. asperellum IIRRCK 1 to 4 (with the accession number: MH825714, MH825715, MH825716, MH825717, respectively) (Kannan et al. 2018) and other 4 Trichoderma isolates from the Department of Plant Pathology, ICAR-IIRR, were used for the study. The axenic cultures were maintained in standard potato dextrose agar (PDA) medium at 4 °C for daily work. Pure cultures grown in PDA at 28 ± 2 °C were subjected to morphological and biochemical analysis. Colony colour, radial growth of the colony after 72 h of incubation at 26 ± 2 °C, nature of conidia and conidiophore, number of chlamydospores, indole acetic acid (IAA) production and phosphate solubilisation were recorded. The mycelial growth of the cultures was observed in a circular pattern, and the radius of the outermost circle from the centre was measured in millimetres. Chlamydospores from 0.5 ml of culture suspension, serially diluted to 10−4 times, were counted on a haemocytometer in a compound microscope (× 400).
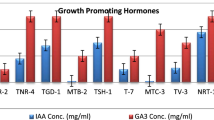
Phosphate solubilising potential of the isolates was estimated from an aliquot of 0.5-ml spore suspension of Trichoderma and cultured in 50-ml National Botanical Research Institute phosphate growth (NBRIP) medium (Nautiyal 1999) and incubated in a shaker at 180 r/min for 7 days at 27 °C. After centrifugation, the supernatant was collected from which the soluble phosphate was quantified, using colorimetric molybdate/antimony method in microgrammes per millilitre (Murphy and Riley 1962). IAA synthesis of the isolate was studied using 0.5-ml spore suspension obtained from the cultures grown in potato dextrose broth (PDB) with tryptophan (200 μg/ml) and was kept for incubation at 180 r/min for 7 days at 27 °C. The filtrates were collected, using Salkowski’s reagent, and estimated using the colorimetric method in microgrammes per millilitre (Gravel et al. 2007).
A phylogenetic tree was prepared to compare the relationship of TAIK-1 with similar isolates and other genera of Trichoderma (Rai et al. 2016). The pathogens, viz., R. solani and S. oryzae, obtained from the Department of Plant Pathology, ICAR-IIRR, were reconfirmed to prove Koch’s postulates in the susceptible variety TN-1.
In vitro screening of bioagents against the pathogens
Biocontrol efficacy of T. asperellum (TAIK 1–4) along with other unidentified Trichoderma isolates against R. solani and S. oryzae was estimated in vitro, using the dual culture method (Sinclair and Dhingra 1995). Inoculum discs from axenic cultures of antagonist and the pathogens were placed simultaneously on the edges of the Petri plate, opposite to each other at equal distance from the centre, and incubated at 28 + 2 °C. A single disc of each pathogen was kept in separate PDA plates to serve as a control. The percentage of mycelial inhibition was evaluated by the formula:
where C = colony growth in centimetres or number of sclerotia in control and T = colony growth in centimetres or number of sclerotia in the particular isolate
In vivo screening of the selected isolates of bioagents against the pathogens under glass house conditions
Isolates of T. asperellum (TAIK-1 to 4), identified from the above in vitro studies, were evaluated in plants grown in pots under glass house conditions. The biocontrol efficacy of the bioagents was correlated with their chlamydospore producing ability in the axenic culture under incubated room conditions. Mud pots of size 30 × 25 cm with 5 kg autoclaved soil were used for the experiments. Seeds of susceptible rice variety TN1 were pre-soaked for 12 h in water mixed with the isolates of T. asperellum @ 20 ml broth/kg of seeds with an inoculum load ranging from 25 to 30 × 106 colony-forming unit (CFU)/ml broth and incubated for 12 h before sowing. The soil was treated with 100 ml broth of each isolate, 30 days after transplanting (DAT) and maintaining the same spore load in the broth as mentioned above. The pathogens were inoculated again at 40 DAT, and the disease severity was recorded for studying the interaction between chlamydospores formed and disease severity.
Assessment of spore induction in T. asperellum using different stimuli
T. asperellum (TAIK-1, 2, 3 and 4), selected from the above screenings, were subjected to different stimuli as follows: (1) near UV blue light of wavelength 400 nm (NUV), (2) normal fluorescent tube light, (3) injury with a scalpel and (4) different nitrogen-enriched PDA media. PDA medium was supplemented by different sources of 0.1% nitrogen, viz., ammonium nitrate (AN), potassium nitrate (PN), peptone (P) and half strength PDA (H) (Steyaert et al. 2010). A control was maintained with normal PDA (PDA). The inoculated plates were exposed to 12 h of alternate NUV light (400 nm) and darkness for 48 h, and the colonies were then subjected to a 1-cm superficial cut with a sterile scalpel at 4 points in the plates to induce conidial formation (Schmoll et al. 2010). Similar conditions were maintained as control for plates exposed to normal light and subsequent injury after 48 h. After 96 h of incubation, CFU was estimated for each treatment.
Evaluation of different organic substrates for bioformulation
Four organic substrates, viz., sorghum grains (SG), rice husk (RH), ragi cob waste (RC) and maize cob waste (MC) that are commonly available, were evaluated for their effectivity on the growth and shelf life of TAIK-1 over a period of 5 months, under room temperature (25 ± 2 °C). The substrates were soaked overnight in water for 12 h, autoclaved, allowed to cool and inoculated with colonies of TAIK-1. Population dynamics of TAIK-1 from the culture bags with different carriers were estimated over a period of 5 months by periodic analysis of CFU at fortnightly intervals. Talc formulation (T) with molasses yeast extract agar medium was used as standard check.
Mass multiplication of test pathogens
Rhizoctonia solani was multiplied on shoot bits of typha (Typha angustata) (Bhaktavatsalam et al. 1978) and S. oryzae on rice husk and rice grain in the ratio of 2:1 (Kumar et al. 2003). Artificial infection of R. solani was created by placing the infected typha shoot bits, with an average count of 8 sclerotia/cm, between the tillers in the central region of the rice hills, 5–10 cm above the water line. Similarly, the infection of S. oryzae was created by applying the infected rice husk containing an average CFU of 106/g, near the base of the hills. Regular examination was done for symptom appearance and sclerotia formation in the respective pots.
Efficiency of TAIK-1 under pot culture conditions
Mud pots of size 30 × 25 cm, each with 5-kg autoclaved soil, were used for the experiments. T. asperellum mass produced in different carriers were used for the study. Pre-soaked seeds were treated with individual isolates of TAIK-1, at the rate of 10 g/kg of seeds, and sown in trays with sterile soil. Well grown were transplanted to the pots after 21 days of sowing. TAIK-1 mass multiplied in different substrates as studied before were applied at the rate of 100 g per pot as soil application at 30 DAT. The pots were watered regularly and weeding done whenever necessary. R. solani or S. oryzae multiplied as above were inoculated 10 days after the soil application of TAIK-1 (40 DAT) and observed for the disease severity, root and shoot dry weight and yield per hill.
Statistical analysis
The experiments were conducted with 3 replications in completely randomised design (CRD), repeated twice, and the means were obtained for the purpose of analysis. Statistical product and service solutions (SPSSR20) software was used for the statistical analysis of all the data obtained. Post hoc test with Duncan’s multiple range test (DMRT) at 5% (P ≤ 0.05) significance level was applied for one-way analysis of variance (ANOVA). Descriptive statistics with homogeneity test was used for three-way ANOVA. Regression relationship between the dependant and independent variables was determined using SPSS (Fig. 1).
Results and discussion
Morphological and biochemical characterisation of Trichoderma
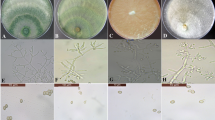
The colonies of different isolates were characterised based on their morphology, IAA production and phosphate solubilisation. Colonies grown in PDA were white and downy initially, later turning into yellowish-green to deep green compact tufts. The conidiophores were profusely branched, verticillate with clusters of flask-shaped phialides bearing green conidia at the terminal end (Plate 1 a, b, c). The isolates solubilised phosphate and synthesised IAA which indicated the efficiency to induce plant growth promotion in rice (Table 1). IAA is one of the most important plant growth hormones involved in the growth and development of the root meristems. IAA produced by Trichoderma spp. helps to increased vigour index of seeds, root architecture, shoot growth and total biomass of the plant (Ortíz-Castro et al. 2009). Similarly, P is a major nutrient required by the plants for their normal functioning and the phosphate solubilising ability of Trichoderma spp., along with several of its growth promoting attributes, plays an important role in improving the overall health of the plants (Bononi et al. 2020). TAIK-1 was found to possess better ability to synthesise IAA and solubilise phosphate and efficient in suppressing the two pathogens of rice.
Phylogenetic analysis of Trichoderma
Molecular characterisation coupled with the studies on morphological characters provided the fool proof identification of the isolates. The phylogenetic tree helped in the comparative genome study of TAIK-1 with other 18 Trichoderma spp. selected based on the sequences of the internal transcribed spacer (ITS1, 5.8 S and ITS2) (Fig. 2).
In vitro bio-efficacy screening of Trichoderma isolates against R. solani and S. oryzae
Rhizoctonia solani and S. oryzae are ubiquitous fungi, opportunistic in nature, that infect a wide variety of plants, and the host plants do not in general possess inherent resistance against them (Rosas et al. 2016). Dual culture screening of isolates of Trichoderma against R. solani and S. oryzae indicated their suppression by Trichoderma. The growth of R. solani under the influence of TAIK-1 was found to be 43.3 mm in 72 h, when compared with 90 mm in control. Similarly, the growth of S. oryzae in the presence of TAIK-1 was 36 mm when compared with 90 mm in control. The percent growth inhibition and percent reduction in sclerotia of the pathogens are given in Table 2. Trichoderma spp. dominate other pathogenic microbes in the rhizosphere by the use of several methods, singly or in a consortia mode, and mycoparasitism coupled with the release of antimicrobial metabolites appears to be the major factors in determining the efficiency of Trichoderma in dual culture assays (Verma et al. 2007). Effector molecules released by R. solani and S. oryzae are recognised by the receptors present on the surface of Trichoderma hyphae, which coil and suck their nutrients which leads to the ultimate death of the hyphae (Elad et al. 1983). Release of extra-cellular antimicrobial metabolites in the culture medium by T. asperellum, which either kill or suppress the growth of the pathogens, has been reported earlier (Akinbode and Ikotun 2008) and also observed by this group (unpublished). The difference in the efficiency of the isolates in suppressing the pathogens is a result of their capacities to parasitize the pathogen colonies or to release such compounds in the media that may deter the growth and development of the pathogen.
In vivo pot culture screening of T. asperellum against R. solani and S. oryzae
Pot culture studies indicated that the TAIK-1 was the most efficient among the 3 isolates and it reduced the severity of sheath blight by 57.25% over control and stem rot by a percentage of 53.25% over control. TAIK 2 was found to be the least efficient among all the isolates, causing 25.43 and 18.67% reduction in the severity of sheath blight and stem rot, respectively. The antagonistic potential of Trichoderma spp. has been reported to be effective against the soil-borne pathogens Rhizoctonia, Sclerotium, Fusarium and Verticilium (Amin and Razdan 2010). Chlamydospores are multiplication propagules produced by some fungi which help them to survive the adverse conditions and to reproduce once the conditions become favourable (Mishra et al. 2012), and thus, the chlamydospore producing efficiency of Trichoderma can be directly related to their better survival and more competitive saprophytic ability. Regression analysis between chlamydospores formed by the isolates of Trichoderma and their disease suppression efficiency indicated that the isolates with better chlamydospore producing abilities were more competent in reducing the disease severity (Fig. 1). Such chlamydospore-efficient strains are also more amenable for commercial formulations, long-term storage and use. In the absence of any stable resistant genes against these two pathogenic fungi, biological control offers a sustainable management solution with least cost to the environment. During off season, antagonistic microbes applied to the soil dominate the soil ecosphere and grow on the pathogen propagules that are overwintering in the crop residues. During cropping season, the antagonists occupy the rhizosphere zone, thereby depriving the pathogens of their space and nutrients. Thus, systematic periodical application of bioagents ensures the continuous presence of the antagonistic populations in the rhizosphere which prevents the build of the pathogen populations in the field.
Conidiation efficiency of T. asperellum against different stimuli
The studies on the effect of different light and/or injury conditions and nitrogen-enriched media on the CFU count (× 106) of the isolates of T. asperellum indicated that there exist significant interactions among these three variables (Supplementary). Among all the different combinations, the isolate TAIK-1 grown on PDA media with peptone (P) with near UV blue light + injury produced significantly the highest CFU count when compared with the combination of TAIK3 on half strength PDA (HPDA) under normal fluorescent tube light, which produced the lowest CFU counts (Table 3). Individually, TAIK-1 was found to be the most efficient strain among the 4 studied, producing the maximum colonies (2.268 × 106), followed by TAIK4 (2.020 × 106) and TAIK3 (1.98 × 106), which were on a par with each other. TAIK2 recorded the lowest CFU (1.712 × 106) (CD: P ˂ 0.05 = 0.053).
The isolates were influenced by different nitrogen sources in the media resulting in varied CFU count. Medium P (2.590 × 106) with peptone as the nitrogen source was found to induce maximum colonies of all the four isolates of T. asperellum, followed by PDA (2.137 × 106), PN (2.07 × 106) and AN (1.958 × 106). The isolates grown on medium HPDA (1.222 × 106), which is a half strength PDA without any nitrogen source, were having the lowest CFU (CD: P ˂ 0.05 = 0.060).
The treatments in which isolates were grown in different light conditions and subjected to injury were also found to significantly differ with respect to the CFU count. The maximum CFU (2.288 × 106) was observed with the treatment condition T4 (NUV light + injury) for all the isolates studied, followed by T3 (2.124 × 106) and T2 (1.875 × 106). The lowest CFU count (1.700 × 106) was noted with T1 (normal light and no injury). It is to be noted that injury to the colonies had a positive effect on the CFU of the isolates (CD: P ˂ 0.05).
Bioformulations would be more viable and effective when dominated with the conidial propagules rather than with the mycelia of the fungi. Accelerated production of conidia reduced the production time, the competition between the actively growing mycelia, and thus ensures better viability of the bioformulation. Fungal colonies produce conidia upon maturity or in response to the stress (Igbalajobi et al. 2019). Fungi respond differently to NUV light, suppresses the conidial formation in Alternaria and Verticillium while inducing the conidial production in Trichoderma. Photo-conidiation as photo-response is conditioned by different photoreceptors in Trichoderma spp. (Schmoll et al. 2010), which induces peptaibol release that enhances their antagonistic efficiency. Further, Osorio-Concepción et al. (2017) indicated that in T. atroviride the blue-light regulator (BLR) proteins BLR-1 and BLR-2 regulate several important activities in the cell including gene transcription and conidial production. Mechanical injury to the colonies provokes a stress response in mycelia that induces NADPH oxidase–dependent reactive oxygen species production and enhancement of calcium signalling mechanisms to trigger the production of conidia (Hernández-Oñate et al. 2012). Activation of phospholipase A2 as a result of lipid metabolism is also a response to mechanical injuries which in turn regulates the production of oxylipins that helps in the regeneration and differentiation of wound tissues resulting in the production of conidia (Dhondt et al. 2000). Nitrogen is one of the most important nutrients in the fungal system, forming the major part of all amino acids which in turn form the base of all proteins and the role of proteins in regulating the metabolic and maturation processes of cells. Differentiation of hyphae from vegetative to reproductive phase and the formation of conidia are directly influenced by the quality and quantity of nitrogen in the media (Silva et al. 2014).
Viability of TAIK-1 in different carriers
Among the 4 different substrates tested, SG was found to support better growth and development of TAIK-1. SG contained the highest conidial count on the 30th day of inoculation, with an increase of 10.05% over RC, 15.49% over RH and 45.37% over MC. A negative correlation was observed between conidial count and the number of days viable conidia survived in different carriers (Fig. 3). CFU of TAIK-1 was higher in the organic substrates in the initial phase of the storage until 30 days and starts to decline thereafter. The colonies obtained from this stage of storage showed a maximum suppression of R. solani and S. oryzae. The decline in CFU upon storage may be due to the fact that TAIK-1 grows profusely because of easily available sugars released by the organic substrates and as the population reaches a peak, the competition for space and nutrients become fatal and the decline phase sets in (Naeimi et al. 2020). Thus, it is obvious that the organic substrates are not suitable for the long-term storage of the biocontrol agents. However, these substrates are environmental friendly, providing organic matter in the soil to support the growth of the saprophytic antagonists. Further, these materials are easily available to the farmers who can use them to multiply the bioagents at their farm level for continuous use and apply as and when required by the crops, thereby reducing their chances of using unregulated spurious products of inferior quality.
Efficiency of disease suppression by TAIK-1 under pot culture conditions
Seeds treated with TAIK-1 germinated early and uniformly when compared to the untreated seeds. After 12 h of soaking with the bioagent, the seeds were found to be colonised by the fungi and germinated uniformly. TAIK-1 cultured in different organic substrates varied in their growth which is indicated by their differing efficiency to suppress the pathogens and their disease development. Increased reduction of the disease severity was an indicator of more growth of TAIK-1 in the particular substrate. Accordingly, TAIK-1 grown on SG was found to be more effective and suppressed the disease severity of R. solani (64.25% reduction over control (ROC)) and S. oryzae (61.29% ROC) with the highest efficiency. This was followed by TAIK-1 grown on T, RC, RH and MC in that order (Table 4). Seed treatment is an effective method of application of the bioagents which increases their chances of growing along with the seedling (Chao et al. 1986), thereby improving the initial vigour and vitality of the seedlings. Pre-soaking the seed in water helps in softening of the lemma and palea which in turn helps the bioagent, TAIK-1, to colonise them and probably may enter the pericarp for non-parasitic endophytic colonisation of the germinating seed (Mishra et al. 2012). However, the ideal duration of pre-soaking the seeds in water and incubating with the bioagents need to be standardised for individual varieties cultivated. TAIK-1 applied on the 30th day of transplanting, coinciding with the tillering stage of rice, releases growth promoting substances that help in the production of maximum functional tillers. The tillering stage is also critical for the infection of R. solani and S. oryzae. The presence of the TAIK-1 during this stage helps in suppressions of the invading pathogens and also makes the plant grow robust and healthy, making them effectively defend themselves against the pathogen incidence and reducing the disease severity.
Further studies are in progress by this group to understand the physiological, biochemical and molecular mechanisms of Trichoderma on rice diseases. Efforts are on to develop suitable formulations, more specifically encapsulated TAIK-1 that helps in its release in a precise, sustained and effective manner.
Conclusion
The results revealed that the isolate TAIK-1 of T. asperellum had the ability to release plant growth hormones and suppress the 2 major rice pathogens, viz., R. solani and S. oryzae. Chlamydospores produced by TAIK-1 help in their survival under stress conditions. Various light, injury and nitrogen sources in the media enhanced the conidia producing ability of TAIK-1. Mass multiplication of organic substrates provided a long-term viability of TAIK-1, which can be considered for commercial storage and use.
Availability of data and materials
Data and materials in this study can be available on reasonable request.
Abbreviations
- TAIK-1:
-
Trichoderma asperellum Indian Institute of Rice Research Kannan1
- IAA:
-
Indole acetic acid
- PDA:
-
Potato dextrose agar
- NBRIP:
-
National Botanical Research Institute phosphate growth medium
- PDB:
-
Potato dextrose broth
- DAT:
-
Days after transplanting
- AN:
-
Ammonium nitrate
- PN:
-
Potassium nitrate
- P:
-
Peptone
- H:
-
Half strength PDA
- SG:
-
Sorghum grains
- RH:
-
Rice husk
- RC:
-
Ragi cob waste
- MC:
-
Maize cob waste
- CFU:
-
Colony-forming unit
References
Akinbode OA, Ikotun T (2008) Evaluation of some bioagents and botanicals in in vitro control of Colletotrichum destructivum. Afr J Biotechnol 7(7):868–872. https://doi.org/10.5897/AJB07.676
Amin F, Razdan VK (2010) Potential of Trichoderma species as biocontrol agents of soil borne fungal propagules. J Phytol 2(10):38–41
Bhaktavatsalam G, Satyanarayana K, Reddy APK, John VT (1978) Evaluation of sheath blight resistance in rice. Int Rice Res Newsl 3:9–10
Bononi L, Chiaramonte JB, Pansa CC, Moitinho MA, Melo IS (2020) Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci Rep 10(1):1–13. https://doi.org/10.1038/s41598-020-59793-81
Chao WL, Nelson EB, Harman GE, Hoch HC (1986) Colonization of the rhizosphere by biological control agents applied to seeds. Phytopathology 76(1):60–65
Deising HB, Reimann S, Pascholati SF (2008) Mechanisms and significance of fungicide resistance. Braz J Microbiol 39(2):286–295
Dhondt S, Geoffroy P, Stelmach BA, Legrand M, Heitz T (2000) Soluble phospholipase A2 activity is induced before oxylipin accumulation in tobacco mosaic virus-infected tobacco leaves and is contributed by patatin-like enzymes. Plant J 23(4):431–440. https://doi.org/10.1046/j.1365-313x.2000.00802.x
Elad Y, Chet I, Boyle P, Henis Y (1983) Parasitism of Trichoderma spp. on Rhizoctonia solani and Sclerotium rolfsii-scanning electron microscopy and fluorescence microscopy. Phytopathology 73(1):85–88
Gnanamanickam SS (2009) Biological control of rice diseases, vol 8. Springer Science & Business Media, Berlin, p 99. https://doi.org/10.1007/978-90-481-2465-7
Gravel V, Antoun H, Tweddell RJ (2007) Growth stimulation and fruit yield improvement of greenhouse tomato plants by inoculation with Pseudomonas putida or Trichoderma atroviride: possible role of indole acetic acid (IAA). Soil Biol Biochem 39(8):1968–1977
Hernández-Oñate MA, Esquivel-Naranjo EU, Mendoza-Mendoza A, Stewart A, Herrera-Estrella AH (2012) An injury-response mechanism conserved across kingdoms determines entry of the fungus Trichoderma atroviride into development. Proc Natl Acad Sci 109(37):14918–14923. https://doi.org/10.1073/pnas.1209396109
Hussain B (2015) Modernization in plant breeding approaches for improving biotic stress resistance in crop plants. Turk J Agric For 39(4):515–530
Igbalajobi O, Yu Z, Fischer R (2019) Red- and blue-light sensing in the plant pathogen Alternaria alternata depends on phytochrome and the white-collar protein LreA. MBio 10(2):1–17. https://doi.org/10.1128/mBio.00371-19
Kannan C, Pradhan B, Renuka R, Anila R, Prakasam V, Prasad MS, Sundaram RM, Sudhakar R (2018) Characterization of native rice specific isolates of Trichoderma and evaluation of its effect on sheath blight pathogen Rhizoctonia solani. J Rice Res 11(1):52–56
Kumar A, Singh RAM, Jalali BL (2003) Evaluation of resistance to stem rot and yield losses caused by the disease in rice. Indian Phytopathol 56(4):403–407
Mishra DS, Prajapati CR, Gupta AK, Sharma SD (2012) Relative bio-efficacy and shelf-life of mycelial fragments, conidia and chlamydospores of Trichoderma harzianum. Vegetos 25(1):225–232
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta 27:31–36. https://doi.org/10.1016/S0003-2670(00)88444-5
Naeimi S, Khosravi V, Varga A, Vágvölgyi C, Kredics L (2020) Screening of organic substrates for solid-state fermentation, viability and bioefficacy of Trichoderma harzianum AS12-2, a biocontrol strain against rice sheath blight disease. Agronomy 10(9):1258
Nagarajkumar M, Bhaskaran R, Velazhahan R (2004) Involvement of secondary metabolites and extracellular lytic enzymes produced by Pseudomonas fluorescens in inhibition of Rhizoctonia solani, the rice sheath blight pathogen. Microbiol Res 159(1):73–81. https://doi.org/10.1016/j.micres.2004.01.005
Nautiyal CS (1999) An efficient microbiological growth medium for screening phosphate solubilizing microorganisms. FEMS Microbiol Lett 170(1):265–270. https://doi.org/10.1111/j.1574-6968.1999.tb13383.x
Ortíz-Castro R, Contreras-Cornejo HA, Macías-Rodríguez L, López-Bucio J (2009) The role of microbial signals in plant growth and development. Plant Signal Behav 4(8):701–712. https://doi.org/10.4161/psb.4.8.9047
Osorio-Concepción M, Cristóbal-Mondragón GR, Gutiérrez-Medina B, Casas-Flores S (2017) Histone deacetylase HDA-2 regulates Trichoderma atroviride growth, conidiation, blue light perception, and oxidative stress responses. Appl Environ Microbiol 83(3):e02922–e02916. https://doi.org/10.1128/AEM.02922-16
Rai S, Kashyap PL, Kumar S, Srivastava AK, Ramteke PW (2016) Identification, characterization and phylogenetic analysis of antifungal Trichoderma from tomato rhizosphere. Springer Plus 5(1):1939. https://doi.org/10.1186/s40064-016-3657-4
Rakesh PS, Ankita P, Annie MC, AshutoshMasih EM, Geethu Rachel GS, Jayajiwan Simon JTG, Schilling P, Lombi KM, Alex R, Balraj V, Mohan VR (2017) Chemical use in farming and its health and environmental implications in a rural setting in southern India. IOSR J Environ Sci: 11;90–95
Rosas JE, Martínez S, Bonnecarrère V, Pérez de Vida F, Blanco P, Malosetti M, Jannink JL, Gutiérrez L (2016) Comparison of phenotyping methods for resistance to stem rot and aggregated sheath spot in rice. Crop Sci 56(4):1619–1627. https://doi.org/10.2135/cropsci2015.09.0598
Schmoll M, Esquivel-Naranjo EU, Herrera-Estrella A (2010) Trichoderma in the light of day–physiology and development. Fungal Genet Biol 47(11):909–916. https://doi.org/10.1016/j.fgb.2010.04.010
Sharma A, Diwevidi VD, Singh S, Pawar KK, Jerman M, Singh LB, Singh S, Srivastawav D (2013) Biological control and its important in agriculture. Int J Biotechnol Bioeng Res 4(3):175–180
Silva RN, Steindorff AS, Monteiro VN (2014) Metabolic diversity of Trichoderma. In: Biotechnology and biology of Trichoderma. Elsevier, Amsterdam, pp 363–376. https://doi.org/10.1016/B978-0-444-59576-8.00027-8
Sinclair JB, Dhingra OD (1995) Basic plant pathology methods. CRC Press, Boca Raton. https://doi.org/10.1201/9781315138138
Steyaert JM, Weld RJ, Mendoza-Mendoza A, Stewart A (2010) Reproduction without sex: conidiation in the filamentous fungus Trichoderma. Microbiology 156(10):2887–2900. https://doi.org/10.1099/mic.0.041715-0
Verma M, Brar SK, Tyagi RD, Surampalli RY, Valero JR (2007) Antagonistic fungi, Trichoderma spp.: panoply of biological control. Biochem Eng J 37(1):1–20. https://doi.org/10.1016/j.bej.2007.05.012
Acknowledgements
The authors acknowledge the ICAR-Indian Institute of Rice Research for the support in the research work conducted. Help rendered in statistical analysis by Dr.C.T. Jose, Dr.J.S. Mishra and Dr.S. Mondal is thankfully acknowledged.
Funding
The authors thank the Indian Council of Agricultural Research, New Delhi, for funding the study.
Author information
Authors and Affiliations
Contributions
All the authors have read and approved the manuscript. Conceptualization and review was done by CK. Data curation, analysis and original draft were maintained by DM. Software handling was done by MA, VP and KB. Formal analysis and administration was handled by MSP and GSJ. Editing was done by RMS.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
No potential conflict of interest was reported by the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Additional file 1.
Three-way Analysis of Variance for CFU count of the isolates under the influence of external stimuli.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chinnaswami, K., Mishra, D., Miriyala, A. et al. Native isolates of Trichoderma as bio-suppressants against sheath blight and stem rot pathogens of rice. Egypt J Biol Pest Control 31, 12 (2021). https://doi.org/10.1186/s41938-020-00356-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41938-020-00356-4