Abstract
Background
The visual system of desert rodents demonstrates a rather high degree of development and specific features associated with adaptation to arid environment. The aim of this study is to carry out a descriptive investigation into the most relevant features of the sand rat eye.
Results
Light microscopic observations revealed that the eye of Psammomys obesus diurnal species, appears similar to that of others rodent with characteristic mammalian organization. The eye was formed by the three distinct layers typical in vertebrates: fibrous tunic (sclera and cornea); vascular tunic (Iris, Ciliary body, Choroid); nervous tunic (retina). Three chambers of fluid fundamentals in maintaining the eyeball’s normal size and shape: Anterior chamber (between cornea and iris), Posterior chamber (between iris, zonule fibers and lens) and the Vitreous chamber (between the lens and the retina) The first two chambers are filled with aqueous humor whereas the vitreous chamber is filled with a more viscous fluid, the vitreous humor. These fluids are made up of 99.9% water. However, the main features, related to life style and arid environment, are the egg-shaped lens, the heavy pigmentation of the middle layer and an extensive folding of ciliary processes, thus developing a large surface area, for ultrafiltration and active fluid transport, this being the actual site of aqueous production. The ciliary muscle is poorly developed and the dilator pupillae is not apparent.
Conclusions
The ocular globe of sand rat demonstrates a high degree of development and several specific features associated with adaptation to life style and arid environment.
Similar content being viewed by others
Background
The lifestyle and type of habitat have an impact on the morphology of the eye. Numerous studies of various species have demonstrated high variability in morphology, especially of the light-sensitive retina, reflecting adaptation to different ecological surroundings and life styles (McFarland, 1991). Adaptation of peculiar habits among different animal species frequently stimulates structural modifications, making species more suitable to a typical environment (Cernuda-Cernuda, Garcia-Fernandez, Gordijn, Bovee-Geurts, & De Grip, 2003; Duke-Elder, 1958). Desert rodents possess physiological and anatomical adaptations to endure the harsch conditions; these habitats have characteristic climatic factors (the heat and the lack of water) affecting probably the structure of the eyes. The morphology of eye has been widely reported for humans (Beuerman & Pedroza, 1996) and various vertebrates, including monkey (Sugita & Nakano, 1980; Svedbergh & Bill, 1972), rat (Yee, Edelhauser, & Stern, 1987) and fish (Soules & Link, 2005). Though there are no published reports describing the ocular morphology of desert rodents. The only literature existing consists on the structure of the retina of Psammomys obesus (Saidi, Chaouacha-Chekir, & Hicks, 2011).The fat sand rat, Psammomys obesus lives in the arid zones of North Africa and Eastern Mediterranean region. P. obesus is the only desert gerbil strictly diurnal and strictly herbivorous, it is independent of any free water; it can maintain a positive water balance without water intake. It prefers the habitats of salt marshes and wadis where halophytic plants are abundant (Degen, 1993). This animal is a unique polygenic animal model for obesity and type 2 diabetes (Kaiser, Cerasi, & Leibowitz, 2012). The present study may provide a fair knowledge concerning the adaptive structure of the eyes in these habitats and could be a tool for biomedical research.
Methods
The investigated animals belonging to Gerbillinae subfamily consisting of several genera represented in this work by Psammomys. The animals were trapped in the desert of Beni-Abbes, region distant of 1250 km from the capital Algiers. They were housed in the animal facility of the Research Unit of dry area (URZA). The animals were cared for in accordance with the criteria outlined in the “Guide for the Care and Use of Experimental Animals” prepared by the National Academy of Sciences and published by the National Institute of Health following approval by the Institutional Animal Care Committee of the Algerian Higher Education and Scientific Research. Seven male and five female adult sand rats, weighing 80–120 g, were anesthetized, by an intraperitoneal injection of 25% urethane solution (0.4 mLper 100 g of body weight), sacrificed, and dissected. The eyeballs were carefully excised and dissected from the surrounding periorbital fat and extraocular muscles. The enucleated eyes were fixed by immersion in a 10% buffered neutral formaldehyde solution, for approximately 1 week, once adequately fixed, the eyes were first dehydrated through graded alcohols, cleared in xylol and immersed in liquid paraffin at 60 °C. Subsequently obtaining the paraffin blocks, 5 μm thick sections were cut and stained with standard Hematoxylin/ Eosin, Masson Trichrome, Van Gieson and Periodic Acid-Schiff (PAS). Histological study was done using light microscope (ZEISS Axoplan) and photographs were taken for detailed illustration of the results. The thickness of cornea and retina was measured using an image analysis program (version 4.6 AxioVision) with 40x objective attached to a light microscope (Zeiss).
Results
The paired eyeballs or globes are situated in the bony sockets of the skull called orbits. The sand rat exhibits a spherical eye shape weigh about 0.45 g. The globe consists of three concentric tunics. The outermost fibrous tunic comprises the cornea in front and the sclera in other parts of the globe (the opaque sclera covers the posterior part of the eye; the transparent cornea the anterior portion). The intermediate vascular tunic consists of the iris, ciliary body, and choroid. The innermost tunica nervosa corresponds to the retina.
The sand rat corneal thickness is about 131, 57 ± 22, 66-μm centrally, it consists of from outside to inside, stratified non-keratinizing squamous epithelium, stroma, Descemet’s membrane, and endothelium. The stroma is the thickest layer of cornea, composed of extremely regularly arranged bundles of collagen fibrils. The Descemet’s membrane is clearly visible, and it corresponds to a true basement membrane of the endothelium. The endothelium is a single layer of flattened cells (Fig. 1). The sclera consisted of dense connective tissue (Fig. 2).
The middle layer contains the choroid, the ciliary body, and the iris. This tunic is richly vascular and densely pigmented. The choroid is the highly pigmented and vascular layer of this tunic, at the ciliary body it’s much thickened (Fig. 2).
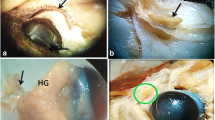
One of the most prominent features of the sand rat eye is the large size and the triangular shape of the ciliary body. It’s the anterior portion of the middle tunic, which is located between the iris and the choroid. Where its apex is contiguous with the choroid and the base close to the iris. The ciliary processes are considerably convoluted providing a large surface area (Fig. 3a). They contain capillaries surrounded by a loose connective tissue covered by a two-layered ciliary epithelium. The epithelium consisted of an inner layer of non-pigmented cells facing the posterior chamber and an outer layer of pigmented cells that were in contact with the connective tissue stroma. Both cells were columnar in shape (Fig. 3b). From the apex of ciliary processes, start the fibers, called zonule of Zinn (or zonular fibers) (Fig. 3c). These fibers connect ciliary body to the lens and have essential role in the process of accommodation. The ciliary muscle is poorly developed.
Ciliary process, lens and Zonular fibers in Psammomys obesus. a General view showing lens (L) and cilliary processes (arrow). Hematoxylin and eosin (H&E). b Magnified view, note the double layered epithelium, consisting of an inner non-pigmented (thin arrow) and outer pigmented layer (thick arrow), overlying large thin-walled vessels (white arrow) within the stroma. H&E. c Zonular fibers (arrow). H&E
The iris is thick, opaque and heavily pigmented; it divides the space between the cornea and the lens into anterior and posterior chambers, respectively, which communicate by a circular aperture, the pupil. Both are filled with a watery fluid called the aqueous humor (Fig. 4a). Structurally the iris has essentially the same layers as in other species of mammals but the intense pigmentation rendered difficult their distinction. The iridal muscles are represented only by constrictor muscle, prominent near the pupillary margin, while the dilator muscle is not apparent (Fig. 4b).
Histological sections through the lens and iris. a Photomicrograph showing an egg-shaped lens (L), iris (thin arrow), pupil (thick arrow), constrictor muscle (green arrow) ciliary processes (Cp) and Aqueous humor (AH).H&E stain. b Structure of the iris (thin arrow), constrictor muscle (green arrow), Aqueous humor (AH) and cornea (C) Van gieson’s stain. c Magnified view of the lens, Lens capsule (LC), anterior epithelium (Epa) and lens fibers (Lf). H&E stain
The Psammomys obesus lens appears non-spherical with a cone-shaped anterior (Fig. 4a). This egg-shaped lens consists of cellular lens fibers surrounded by a thick basement membrane, the lens capsule covered by a monolayer of epithelium at its anterior surface (Fig. 4c). It has no blood supply but is nourished by the aqueous humor. Thus, this structural organization maintain transparency of the lens to providing refractive power and so, helps to form a focused image on the retina.
The retina represents the internal tunic of the globe. The sand rat retina is about 133, 12 ± 51, 21 μm thickness, arranged into layers according to the standard structure of the mammalian eye. It was divided into: pigment epithelium layer, photoreceptor cell layer, outer limiting membrane, outer nuclear layer, outer plexiform layer, inner nuclear layer, inner plexiform layer, ganglion cell layer and nerve fiber layer (Fig. 5a). The retina transmits the perceived light via the optic nerve to the brain, where visual perceptions are converted into images.
Light photomicrographs showing the organization of the retina and the optic nerve of Psammomys obesus. a Microscopic section of retina. Choroid(C); Retinal pigment epithelium (RPE); photoreceptor (PR) outer segments; outer nuclear layer (ONL); outer plexiform layer (OPL); inner nuclear layer (INL); inner plexiform layer (IPL); ganglion cell layer (G); inner limiting membrane (ILM) and vitreous humor (VH).PAS/hematoxylin staining. b Histology of the optic nerve head. Longitudinal section: optic papilla (arrowhead), artery (thick arrow), vein (thin arrow), Lamina cribrosa (Lc), retina (R) and sclera (S).Van gieson’s stain
The sand rat eye has a large optic nerve might be due to the great number and size of optic fibers that originate from the ganglion cells of the retina. They leave the retina by widely separated optic papillae and pierce the sclera at the lamina cribrosa. The number of its fibers gives a precise assessment of the visual efficiency of the eye. On sectional view (Fig. 5b), Psammomys obesus optic nerve shows clearly that it shares similarities with primate’s optic nerve. The central retinal artery and vein traverse the optic nerve; at the level of the lamina cribrosa, the axons within the optic nerve become myelinated, thus, its diameter doubles. Externally, the optic nerve is surrounded by dura, arachnoid, and pia mater.
Discussion
Psammomys obesus is diurnal desert rodent that inhabits arid and semiarid regions. It’s continuously facing to extremes climatic conditions ((temperature highs of above 50 °C), dry (near zero relative humidity) and dusty (sand storms lasting for days) (Daly & Daly, 1975). The sand rat eye is similar to most mammalian specie’s eye, though there appear to be some specific structural and functional adaptations in each of these species. The eyeball weight in this study was 0.45 g. This is greater than 0.27 g in Australian hopping mouse (Notomys alexis) (Smith, 1976), and 0.014–0.024 g in different strains of mice (Zhou & Williams, 1999). But less than 3.00 g in rabbits (Gelatt, 1981), and 0.99 g in guinea pigs (Latimer, 1951).
The cornea of the studied species was composed of well-defined layers, including a squamous corneal epithelium, the stroma, and corneal endothelium, Such general structure is comparable to what has been described for the corneas of most vertebrate classes including human and other rodents (Beuerman & Pedroza, 1996; Collin & Collin, 1998; Chakravarti, 2001; Svaldeniene, Babrauskienė, & Paunksnienė, 2003).
According to Scott and Bosworth (1990) corneal thickness increased with increasing body size. The cornea in this study was 131, 57 ± 22, 66 μm. It was much thicker than in mouse (approx. 80 μm) and Frog (approx. 45 μm) (Scott & Bosworth, 1990) and noticeably thinner than sheep 500 μm (Prince, Diesem, Eglitis, & Ruskell, 1960) and man 550 μm (Fatt, 1978).
The lens was non-spherical as described by Lluch, Ventura, and López-Fuster (2008) in Arvicolinae. These authors indicate that’ Arvicolinae have larger posterior nodal distances than Murinae. This gives them higher visual resolution in daylight conditions. In addition, the front surface of the lens has a protuberance of increased curvature. Similarly, to what was observed by Mass and Supin (2007) in the Sea Otters, suggesting that the curvature of the lens surface provides sufficient refractive power to focus images on the retina. Moreover, a distinctive characteristic of the eye anatomy is that the iris is fastened to the frontal lens surface. In agreement with Mass and Supin (2007), contraction of iris muscles influences the curvature of the frontal lens surface. This mechanism can provide an accommodation range. This agrees with our current study that the ciliary muscle is poorly developed in sand rat, and as mentioned by Dral (1972) in these conditions’ accommodation cannot be achieved by a change of the lens shape. In accordance to Junqueira and Carneiro (2004), the lens is held in position by a system of radially oriented fibers, called the ciliary zonule. The iris in this species is heavily vascularized and pigmented. Having a well apparent constrictor muscle or pupillary sphincter and the dilatators muscle seem lacking. According to Lluch et al. (2008) both diurnal and arrhythmic mammals, such as the arvicolines studied here, have a better-developed constrictor muscle and a considerably higher amount of melanin granules in the retinal pigment epithelium than nocturnal or crepuscular mammals such as the murines. According to Junqueira, Carneiro, and Kelly (1998), heavy pigmentation of the iris in its anterior and posterior epithelia as well as in the stroma has been postulated to prevent the passage of the light into the interior of the eye except through the pupil.
One of the most prominent features of the sand rat eye is the large size of the ciliary processes highly convoluted. Such microanatomical features are present to enlarge the total epithelial surface area for enough production. It is commonly accepted that the ciliary epithelium is engaged in the production of the aqueous humor. However, their structural characteristics are similar to those described by Čech (2004) in small laboratory mammals (hamsters, guinea-pigs and mice).
The choroid is the highly pigmented and vascular layer of the uveal tunic that underlies most of the eyeball’s surface. According to Sattari, Asli, Mansoori, Kheirandish, and Yavari (2012), it acts as a blood supply system that is made up of several thousand closely arrayed and parallel arterial and venous capillaries. The choroid is also heavily pigmented in most animals. As reported by Koskela, Reiss, Brubaker, and Ellefson (1989) in golden spiny mice, this intense pigmentation may result from the fact that their eyes can withstand intense solar radiation.
The retinal layers are built according to the classic structure of the mammalian eye. The total retina thickness is about 133, 12 ± 51, 21 μm, less than in guinea pig 140 μ, marsupial brushtail possum 170 μ and rat 220 μ (Buttery, Hinrichsen, Weller, & Haight, 1991). In more recent reports by Lluch et al. (2008), it has been stated that the neuroretina is significantly thicker in Murinae than in Arvicolinae. This is probably due to the mainly nocturnal activity patterns of murines. Thus, Murinae retinas are thicker because they contain a greater number of long and slender rods in the external layers of the neuroretina to increase its sensitivity to light. In addition, previous experiments by Saidi, Chaouacha-Chekir, and Hicks (2012) have shown the retinal phenotype of a diurnal rodent Psammomys obesus, with a special emphasis on cone and horizontal cells. The large numbers, size and expression patterns of these two populations indicate the visual system is adapted to diurnal activity, like man.
Our histological data on the optic nerve of Psammomys obesus have shown a comparable composition to that of the monkey, the cat and the dog (Albrecht, 2008).It contains a well-developed lamina cribrosa as reported in human by Jeffery et al. (1995).
Conclusions
We conclude that the main histological features of Psammomys obesus eye are adaptive for diurnal activity and for survival in a desert environment. This species provides an ideal opportunity to understanding pathophysiology of many ocular diseases in human.
References
Albrecht, M. C. (2008). Comparative anatomy of the optic nerve head and inner retina in non-primate animal models used for Glaucoma research. The Open Ophthalmology Journal, 2, 94–101.
Beuerman, R. W., & Pedroza, L. (1996). Ultrastructure of the human cornea. Microscopy Research and Technique, 33, 320–335.
Buttery, R. G., Hinrichsen, C. F. L., Weller, W. L., & Haight, J. R. (1991). How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature. Vision Research, 31, 169–187.
Čech, S. (2004). An attempt to describe the ultrastructure and ultrahistochemistry of ciliary processes in mammals. Biomedical Papers, 148(2), 201–202.
Cernuda-Cernuda, R., Garcia-Fernandez, J. M., Gordijn, M. C., Bovee-Geurts, P. H., & De Grip, W. J. (2003). The eye of the African mole rat Cryptomys anselli: to see or not to see? The European Journal of Neuroscience, 17, 709–720.
Chakravarti, S. (2001). The cornea through the eyes of knockout mice. Experimental Eye Research, 73, 411–419.
Collin, S. P., & Collin, H. B. (1998). A comparative study of the corneal endothelium in vertebrates. Clinical and Experimental Optometry, 81(6), 245–254.
Daly, M., & Daly, S. (1975). Behaviour of Psammomys obesus (Rodentia: Gerbillinae) in the Algerian Sahara. Zeitschrift für Tierpsychologie, 37, 298–321.
Degen, A. (1993). Energy requirements of the fat sand rat (Psammomys obesus) when consuming the salt bush, Atriplex halimus. Journal of Basic and Clinical Physiology and Pharmacology, 4, 13–28.
Dral, A. D. G. (1972). Aquatic and aerial vision in the bottle-nosed dolphin. Netherlands Journal of Sea Research, 5, 510–513.
Duke-Elder, S. (1958). The eye in evolution. In Duke-Elder (Ed.), System of ophthalmology, (vol. 1). London: Henry Kimpton.
Fatt, I. (1978). Physiology of the eye. In An introduction to the vegetative functions, (pp. 123–163). Boston: Butterworth-Heinemann Ltd.
Gelatt, K. N. (1981). Veterinary ophthalmology, (1st ed., ). London: Lea and Febiger.
Jeffery, G., Evans, A., Albon, J., Duance, V., Neal, J., & Dawidek, G. (1995). The human optic nerve: fascicular organisation and connective tissue types along the extra-fascicular matrix. Anatomy and Embryology, 191(6), 491–502.
Junqueira, L. C., & Carneiro, J. (2004). Histologia Básica, (10th ed., p. 488). Rio de Janeiro: Guanabara Koogan.
Junqueira, L.C., Carneiro, J., & Kelly, R.O. (1998). Basic histology, 9. Appleton & Lange, a Simon & Schuster Company, Rio de Janeiro.
Kaiser, N., Cerasi, E., & Leibowitz, G. (2012). Diet-induced diabetes in the sand rat (Psammomys obesus). Methods in Molecular Biology, 933, 89–102.
Koskela, T. K., Reiss, G. R., Brubaker, R. F., & Ellefson, R. D. (1989). Is the high concentration of ascorbic acid in the eye an adaptation to intense solar irradiation? Investigative Ophthalmology and Visual Science, 30, 2265–2267.
Latimer, H. B. (1951). Weight of the eyeballs in Guinea pigs. The Anatomical Record, 110(3), 349–357.
Lluch, S., Ventura, J., & López-Fuster, M. J. (2008). Eye morphology in some wild rodents. Antomia Histologia Embryologia, 37, 41–51.
Mass, A. M., & Supin, A. Y. (2007). Adaptive features of aquatic mammals’ eye. The Anatomical Record, 290, 701–715.
McFarland, W. N. (1991). The visual world of coral reef fishes. In P. F. Sale (Ed.), The ecology of fishes on coral reefs, (pp. 16–38). San Diego: Academic Press.
Prince, J. H., Diesem, C. D., Eglitis, I., & Ruskell, G. L. (1960). The Anatomy of the Eye and Orbit in Domestic Animals. Springfield: Charles C. Thomas.
Saidi, T., Chaouacha-Chekir, R. B., & Hicks, D. (2011). Diurnal rodents as animal models of human central vision: characterisation of the retina of the sand rat Psammomys obsesus. Graefe’s Archive for Clinical and Experimental Ophthalmology, 249, 1029–1037.
Saidi, T., Chaouacha-Chekir, R. B., & Hicks, D. (2012). Advantages of Psammomys obesus as an animal model to study diabetic retinopathy. Journal of Diabetes and Metabolic Disorders, 3, 207.
Sattari, A., Asli, M., Mansoori, F. S., Kheirandish, R., & Yavari, H. (2012). Histological study of middle layer of rabbit fish eye (siganus javus). Asian Pacific Journal of Tropical Biomed, 52, 1086–1089.
Scott, J. E., & Bosworth, T. R. (1990). A comparative biochemical and ultrastructural study of proteoglycan-collagen interactions in corneal stroma. Biochemical Journal, 270, 491–497.
Smith, C. J. D. (1976). Gross anatomical and microscopic observations on the orbit and its content in Notomys alexis. Australian Journal of Zoology, 24, 479–489.
Soules, K. A., & Link, B. A. (2005). Morphogenesis of the anterior segment in the zebrafish eye. BMC Developmental Biology, 5, 12.
Sugita, A., & Nakano, H. (1980). Surface ultrastructure of the transition zone between the cornea and the trabecular meshwork in the Japanese monkey. Nippon Ganka Gakkai Zasshi, 84, 1759–1764.
Svaldeniene, E., Babrauskienė, V., & Paunksnienė, M. (2003). Structural features of the cornea: light and electron microscopy. Veterinarija ir zootechnikat, 24(46), 50–55.
Svedbergh, B., & Bill, A. (1972). Scanning electron microscopic studies of the corneal endothelium in man and monkeys. Acta Ophthalmologica, 50, 321–336.
Yee, R. W., Edelhauser, H. F., & Stern, M. E. (1987). Specular microscopy of vertebrates corneal endothelium: a comparative study. Experimental Eye Research, 44, 703–714.
Zhou, G., & Williams, R. W. (1999). Mouse models for the analysis of myopia: an analysis of variation in eye size of adult mice. Optometry and Vision Science, 76(6), 408–418.
Acknowledgments
The authors thank the personnel of the Research Station of Dry Land of Beni-Abbes.
Funding
The research is funded by Ministry of Higher Education and Research.
Availability of data and materials
Data sharing not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Contributions
OS-B conceived and designed the study, NH prepared the figures and SL revised the manuscript. All authors have participated in the elaboration of this work and have approval for this submission.
Corresponding author
Ethics declarations
Ethics approval
All animals were cared for in accordance with the criteria outlined in the “Guide for the Care and Use of Experimental Animals” prepared by the National Academy of Sciences and published by the National Institute of Health following approval by the Institutional Animal Care Committee of the Algerian Higher Education and Scientific Research.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Saadi-Brenkia, O., Hanniche, N. & Lounis, S. Microscopic anatomy of ocular globe in diurnal desert rodent Psammomys obesus (Cretzschmar, 1828). JoBAZ 79, 43 (2018). https://doi.org/10.1186/s41936-018-0056-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41936-018-0056-0