Abstract
The kidney requires large amount of energy to regulate the balance of fluid, electrolytes and acid-base homeostasis. Mitochondria provide indispensible energy to drive these functions. Diverse energy sources such as fatty acid and glucose are fueled for ATP production at different renal sites controlled by a fine-tuned regulation mechanism. microRNAs (miRNAs) have been implicated in the pathogenesis of various kidney diseases. Recent studies have highlighted their contributions to metabolic abnormalities. Characterization of the miRNAs in renal metabolic disorders may promote a better understanding of the molecular mechanism of these diseases and potentially serve as therapeutic targets.
Similar content being viewed by others
Introduction
The kidney requires a large amount of energy to enable the reabsorption of nutrients and regulation of electrolytes, fluid and acid-base balance. Maintenance of metabolic homeostasis is critical to functioning of the kidney and possibly requires a fine-tuned regulation mechanism. Global analysis has demonstrated that various metabolic disorders are corrected with the alternation of microRNA (miRNA) expression profile, suggesting vital roles of miRNAs in maintaining organ energy homeostasis.
miRNAs are small non-coding RNAs of ~ 22 nucleotides that regulate gene expression at the post-transcriptional level. miRNA is transcribed from intergenic, intronic or polycistronic loci as precursor RNAs (pri-miRNA) in canonical biogenesis pathway [1]. The stem-loop structure from the pri-miRNA is processed by Drosha and DGCR8 or the nuclear spliceosome apparatus. As an alternative way, miRNAs are non-canonically transcribed as endogenous short hairpin RNAs (shRNAs) or derive through splicing from introns (mirtrons) [2]. Then the pre-miRNA are transported to the cytosol by exportin-5 and Ran-GTP-dependent processes and are further processed by complex of RNase III, Dicer and TRBP to form the mature miRNA. miRNA duplex is then unwind by argonaut proteins (Ago2) and incorporates into the argonaut-containing RNA-induced silencing complex (RISC). The RISC-miRNA assembly is then guided to specific target sequences in mRNAs chiefly located in the 3’UTR by Watson-Crick base-pairing of nucleotides 2–8 in the mature miRNA, also called the seed sequence [3].
In this review, we briefly introduce the metabolic feature of the kidney and then discuss the advances in understanding the emerging roles of miRNAs in modulating metabolic disorders, particularly on mitochondrial homeostasis, lipid and glucose metabolism.
Metabolic characterizations of the kidney
The kidney functions to remove waste and regulate fluid and electrolyte balance. The active reabsorption of glucose, sodium and other metabolites from glomerular filtrate is a power task [4,5,6] that makes the kidney one of the most energy-demanding organ and the highest resting metabolic rates in our body [7]. To provide sufficient energy, the kidney is equipped with the highest mitochondrial content and consumes most of the oxygen only secondary to the heart [8, 9]. Moreover, the proximal convoluted tubular cells and the thick ascending loop of Henle (TAL) cells in the kidney cortex contain the majority of the renal mitochondria [10,11,12,13,14] which use the majority of kidney consumed oxygen to generate ATP [4,5,6].
In healthy conditions, large quantities of the renal ATP are produced within the mitochondria via oxidative phosphorylation (OXPHOS) [5, 14, 15]. Electrons from NADH and FADH2 produced by tricarboxylic acid (TCA) cycle are transferred to complex I and complex II, respectively and then through complex III to complex IV to be accepted by oxygen. Concurrently, protons are pumped across the membrane through complexes I, III and IV, and ultimately, flow through complex V (ATP synthase) to drive the production of ATP from ADP.
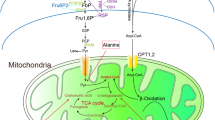
Different renal sites have diverse fuel preference (Table 1). The tubulointerstitial compartment except the glomeruli, utilize free fatty acid (FFA) via β-oxidation and ketone oxidation for ATP generation [16]. Glucose oxidation is preferred in the TAL and the glomerular cells. Whereas, glucose anaerobic metabolism occurs in the more hypoxic renal medulla [17]. Aerobic metabolism of a single molecule of glucose produces 36 molecules of ATP which is more efficient than the production of 2 molecules of ATP by anaerobic metabolism [17]. The FFA oxidation, such as a molecule of palmitic acid produces 106 molecules of ATP, is even more efficient [17]. It is worth note that proximal tubular cells (PTCs) produce glucose from lactate, glutamate and glycerol via gluconeogenesis [18, 19] that also require ATP [20, 21]. The ATP is also required for glomerular filtration and for the synthesis of hormone and proteins, although ATP for these processes are much lower than the reabsorption [7]. The fuel preferences tend to reflect the energy demands at different renal sites in the physiological conditions. The ATP production and energy source is actually flexible. Glomerular cells, including podocytes, endothelial cells and mesengial cells have the ability of aerobic and anaerobic respiration in basal cell processes [22,23,24,25]. In the absence of glucose, amino acid can be alternatively utilized to generate pyruvate to fuel glycolysis and OXPHOS [26, 27] (Fig. 1).
Oxidation of the substrates for energy production in renal mitochondria. Free fatty acids, ketones, glucose, lactate and glutamine are renal cell fuels. They are used for mitochondrial ATP production through the TCA cycle and OXPHOS. ANT, adenine nucleotide translocase; CPT1, carnitine palmitoyltransferase 1; CPT2, carnitine palmitoyltransferase 2; GAT, mitochondrial glutamate transporter; MPC, mitochondrial pyruvate carrier
Taken together, many renal cells have high metabolic rates and are highly dependent on mitochondrial generation of ATP to maintain their physiological morphology and functions.
miRNA regulates lipid metabolism
Fatty acid is one of the major energy sources of the kidney similarly to the heart [16, 28]. The key components of fatty acid oxidation are targets of various miRNAs. Carnitine palmitoyltransferase 1α (CPT1α) mediates the entrance of fatty acid to mitochondria [29], which has been shown to be targeted by miR-33 family [30, 31] and miR-370 [32]. miR-142 targets CPT1α to regulate metabolic reprogramming during immunogenic response [33].
Carnitine ctanoyl transferase (CROT) is a peroxisomal enzyme that allows short chain fatty acid to enter into the mitochondria [29]. miR-33a, miR-33b and the complementary strand miR-33a-3p has been found to target CROT and therefore affect fatty acid β oxidation [30, 31, 34]. Moreover, the intronic region of sterol-regulatory element binding proteins (SREBP2) [35] and SREBP1 [36] genes encode miR-33a and miR-33b, which also targets the 3-ketoacyl-coA thiolase to regulate fatty acid oxidation [31]. In addition, miR-33a and miR-33b was found to target sirtuin SIRT6 [37], a NAD+-dependent histone deacetylase [38,39,40,41]. miR-33 inhibits SIRT6 and leads to acetylation of SREBP1 targeted acetyl-coA carboxylase 1 (ACC1), stearoyl-coA desaturase 1 and fatty acid synthase (FASN), which results in repression of lipogenesis [31].
miR-122 antisense significantly reduces plasma cholesterol level [42, 43]. Transfecting of miR-122 reduces the transcription of aldolase-A in hepatocarcinoma cell line [42]. Pantothenate kinase 1 (PANK) is involved in the synthesis of coenzyme A, which is a cofactor in lipid metabolism [44]. In the intronic sequence of the PANK1α gene locates the miR-103 and miR-107 which affects lipid metabolism [45]. miR-224 targets acyl-coA synthetase long chain family (ACSL4) [45] and alters fatty acid oxidation [46].
Gene expression profiling identifies the upregulation of a group of lipid metabolic genes in the absence of miR-21, including the direct target of miR-21, peroxisome proliferator activated receptor α (PPARα) [47]. miR-21 promotes renal fibrosis by targeting PPARα and Mpv171 to silence lipid metabolic pathway and aggravates ROS generation, respectively [47]. Moreover, miR-21 silencing enhances PPARα/retinoid X receptor and the downstream pathways that protects mitochondrial function and relieves inflammation and fibrogenesis in renal tubule and glomeruli [48]. miR-17 is identified as a novel target for treatment of autosomal dominant polycystic kidney disease (ADPKD), which is the downstream of c-myc and inhibit OXPHOS and stimulate proliferation to aggravate cyst growth via directly repress of PPARα [49]. Similarly, miR-105 regulates the sustained cell growth by targeting MYC [50].
PPARδ mediates the metabolic switch from fatty acid oxidation to glycolysis [51]. miR-199a targets PPARδ to increase lipid accumulation and affects mitochondrial content in heart and liver [52]. PPARδ is also the target of miR-29a [53].
AMP-dependent kinase (AMPK) signaling and insulin receptor signaling pathways are critical cellular energy pathways such as lipid and glucose metabolism [54]. AMPKα1 is targeted by miR-33a and miR-33b [37, 55], which mediates the inhibition of SREBP or phosphorylation and deactivation of SREBP-targeted ACC1 [56, 57]. The insulin receptor substrate 2 (IRS2), one of the adaptor proteins that relays insulin receptor signaling to the downstream effectors, is also the target of miR-33 [37]. Reduced IRS2 and compensatory elevation of IRS1 activates SREBP1 [58], which explains the effect of miR-33 on lipid deposition and hepatosteatosis.
In summary, these results suggest an integrated and extensive interaction between the targets and their miRNAs to regulate lipid metabolism (Fig. 2).
miRNA regulation of lipid metabolism. A schematic of miRNA-regulatory network in lipid metabolism. ACSL4, acyl-coA synthetase long chain 4; AMPKα1, AMP-dependent kinase α1; CPT1α, carnitine palmitoyltransferase 1α; CROT, carnitine ctanoyl transferase; IRS, insulin receptor substrate; PANK, pantothenate kinase; PPAR, peroxisome proliferator activated receptor; SREBP, sterol-regulatory element binding proteins
miRNA modulates glucose metabolism and glycolysis related signaling pathways
Several miRNAs regulates the tissue responses to insulin and glucose metabolism. In diabetes, miR-29a and miR-29b are upregulated in muscle and liver [59], which repress insulin signaling stimulation protein caveolin 2 (CAV2) [60, 61], SREBP negative regulator insulin-induced gene 1 (INSIG1) and insulin intermediate PI3 kinase subunit p85α [59]. miR-126 targets IRS1 to induce insulin signaling inhibition [62]. miR-223 inhibit glucose uptake in skeletal muscle by targeting glucose transporter GLUT4 [63]. miR-103 and miR-107 are probably therapeutic targets for relieving insulin resistance [64]. They affect the availability of insulin receptor by targeting CAV1 [65]. Interestingly, miR-103 and miR-107 are inhibitors of Dicer and their effects are also presumably mediated via other miRNAs [66]. miR-143 is high in diabetic db/db mice and contributes to the reduced insulin signaling sensitivity possibly by targeting Akt related oxysterol-binding protein-related 8 (ORP8) [67]. let-7 miRNA family, also increased in diabetic mice probably results in impaired insulin signaling through targeting insulin-like growth factor 1 receptor (IGF1R) and IRS2 [68].
In proliferative cells such as tumor, several miRNAs have been found to directly target enzymes and transporters involved in the process of glycolysis. Downregulation of miR-106a results in de-repression of GLUT3 and promotes glycolysis [21, 69, 70]. Similarly, downregulation of miR-195-5p leads to de-repression of its target GLUT3 and increases the uptake of glucose in bladder cancer [71]. miR-144 targets GLUT1 which results in reduced glucose uptake and lactate production in lung cancer cells [72]. GLUT1 is also the target of miR-1291 and miR-328 in renal cell carcinoma [73] and colon cancer cell [74], respectively.
The glycolytic enzyme hexokinase 2 (HK2) is the direct target of miR-143 [75]. In addition, HK2 is indirectly regulated by miR-124 and miR-155 both via STAT3 [76, 77]. miR-128, miR-135 and miR-320 target phosphofructokinase (PFK) which is downregulated in lung cancer [78,79,80]. SIRT2 specifically targeted by miR-200c is a critical regulator of several glycolytic enzymes, including aldolase, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), phosphoglycerate kinase (PGK), and enolase [81].
Pyruvate kinase type M2 (PKM2) is targeted by let-7a [82]. Moreover, c-Myc targeted by let-7, is also the activator of hetergenous nucler ribonucleoprotein A1 (hnRNPA1) splicing factor, which in turn downregulates let-7 and forms a positive feedback loop consisting of let-7a/c-Myc/hnRNPA1/PKM2 [82]. PKM2 is also the target of miR-326 in regulation of cell proliferation [83]. PKM2 is targeted by miR133a/b in tongue squamouse cell carcinoma [84,85,86]. The PKM2 targeted by miR-122 is shown to induce metabolic switch from glycolysis to OXPHOS [87]. miR-340, miR-124 and miR-137 target the alternative splicing proteins hnRNPI/hnRNPA1/hnRNPA2, which make the PK PKM2 [88]. miR-26a targets pyruvate dehydrogenase protein X (PDHX) to promote glycolysis and repress OXPHOS [89].
miR-34 targets lactate dehydrogenase A (LDHA) and is also reduced in breast cancer [90, 91]. LDHB is the target of miR-375 [92,93,94]. miR-124 and miR-342-3p target lactate monocarboxylate transporter 1 (MCT1) to inhibit the transport of lactate from cytosol to extracellular space [95, 96].
Besides insulin receptor signaling, glycolytic metabolism is also regulated by receptor tyrosine kinases (RTKs) and the downstream effecter pathways, including c-Met, platelet-derived growth factor receptor α (PDGFRA), epidermal growth factor receptor (EGFR), RAS pathway, PI3K/Akt, mTOR and c-myc. c-Met is targeted by miR-410 [97], miR-144-3p [98], and miR-34a [99,100,101,102]. In addition, miR-34a also targets PDGFRA [102]. miR-128 targets PDGFRA and EGFR [103]. Furthermore, EGFR is the target of miR-219-5p [104, 105] and miR-7 [106, 107].
miR-9-targeted NF1 is the antagonist of RAS [108]. N-RAS is the target of miR-143 [109] and miR-340 [110, 111]. K-RAS is targeted by let-7a [112] and miR-134 [113]. Most of the miRNAs are aforementioned as glycolytic targeting miRNAs, suggesting a strong correlation between RAS and glycolysis.
Activation of PI3K/Akt pathway contributes to the enhanced glycolysis. miR-7 directly targets PI3K [114]. The downstream Akt is targeted by miR-542-3p [115]. miR-21 indirectly regulates PI3K through targeting its antagonist PTEN [116]. Moreover, PTEN is the target of miR-26a [117], miR-1908 [118], miR-494-3p [119], miR-10a/b [120], and miR-21/221 [121, 122].
The PI3K/Akt downstream pathway mTORC1 is the promoter for glycolysis and negatively regulated by AMPK. mTORC1 is indirectly regulated by miR-451 via targeting CAB39, which binds the AMPK activator LKB1 [123, 124]. miR-199a-3p targets mTORC1 and mTORC2 [125]. miR-34a suppresses Rictor, which is the binding partner of mTORC2 [101, 126].
c-Myc is regulated by mTORC2 via FoxO3a and is directly targeted by miR-34c [127]. Interestingly, FoxO3a positively regulates miR-34c [127]. On the contrary, FoxO3a is the target of miR-155 [128].
In conclusion, multiple miRNAs have been shown to affect glucose homeostasis (Fig. 3) and insulin signaling pathway (Fig. 4). The regulatory loops composed of miRNA/glycolysis related signaling pathways/glycolysis are possibly universal in proliferative cells.
miRNA regulation of glycolytic enzymes and transporters. A schematic of miRNA-regulatory network in glycolysis. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; GLUT, glucose transporter; HK2, hexokinase 2; hnRNPA, hetergenous nucler ribonucleoprotein A; PDHX, pyruvate dehydrogenase protein X; PFK, phosphofructokinase; PGK, phosphoglycerate kinase; PKM2, pyruvate kinase type M2; LDH, lactate dehydrogenase; MCT1, monocarboxylate transporter 1
miRNA regulation of glycolysis related signaling pathways. A schematic of miRNA-regulatory network in glycolytic signaling pathways. AMPK, AMP-dependent kinase; CAV, caveolin; EGFR, epidermal growth factor receptor; IGF1R, insulin-like growth factor 1 receptor; INSIG1, insulin-induced gene 1; ORP8, oxysterol-binding protein-related 8; PDGFRA, platelet-derived growth factor receptor α
miRNA in amino acid metabolism
Synthesis and breakdown of amino acid are mainly occurs within the mitochondria. The amino acid is also the energy source of renal tubular cells [16]. Previous studies have shown that amino acid metabolism is regulated by multiple miRNAs. miR-193b regulates serine hydroxyl transferase (SHMT2), which converts serine to glycine [129]. miR-23a and miR-23b have been implicated in proliferative cells to control the expression of glutaminase in mitochondria [130]. Interestingly, their downregulation following c-myc overexpression is also observed during sustained cell proliferation and transformation [130]. The target of miR-29b, digydrolipoyl branched chain acyltransferase is one of the components of branched chain α-ketoacid degydrogenase, which mediates the catabolism of leucin, isoleucine and valine [131].
miRNA modulates the mitochondrial homeostasis
mitomiRs and mitochondria
miRNAs that locate inside the mitochondria are termed mitomiRs, either encoded by the mitochondrial genome or transported into the organelle [132, 133]. miRNAs are not expressed in cells without mitochondrial DNA (mtDNA) suggests that human and mouse mitochondrial genome could encode miRNAs [134]. Moreover, the presence of pre-miR and the corresponding mature miRNAs in mitochondria suggests that miRNA processing may occur in the mitochondria. It is possible that nuclear-encoded miRNAs may be imported into mitochondria [133, 135, 136] where to regulate mtDNA translation [135]. MitomiRs have distinguishable characteristics that separate them from cytosolic miRNA, such as an unusual size between 17 and 25 nt and unique thermodynamic features, which are speculated to facilitate their entry to mitochondria [136]. Multiple putative mitomiR binding sites were revealed on the mtDNA in silico studies [133]; however, evidence showing the import of miRNA into mitochondria is still lacking. Isolation of mitochondria without the contamination of other membrane vesicles remains the major technical obstacle and interpretation of the data should be taken with caution. Whether mitochondria-produced miRNA can be exported to the cytoplasm is still controversial. The mitochondrial-like transcripts probably come from mitochondrial genome equivalents within the nuclear genome [137,138,139].
Evidence of mitomiRs in renal cells remains poorly noticed. The muscle-specific miR-1 enhances mtDNA-encoded transcripts inside the mitochondria of cardiac and skeletal muscle [135]; however, the direct evidence showing the binding of miR-1 to mitochondrial transcripts was lacking. It is also interesting because the translational stimulation effect of miRNAs was merely reported previously. The rat cardiac-specific mitomiR, miR-181c is enriched 2-fold in mitochondria compared to the whole heart, which targets the mRNA of cytochrome c oxidase subunit I (COX1) and regulates mitochondrial respiration [140]. In addition, administration of miR-181c regulates mitochondrial genes and leads to cardiac dysfunction [141]. More reports indicate the role of miR-181a in regulation of mitochondrial apoptosis pathway [142]. In cisplatin-induced acute kidney injury (AKI), repression of mitochondrial resident protein Bcl-1 by miR-181 leads to proximal tubular cells injury [143]. Recent research reveals a panel of aging-related mitomiRs (let7b, miR-146a, −133b, −106a, −19b, −20a, −34a, −181a and − 221) targets a number of mitochondrial resident proteins besides Bcl-1 [144]. miR-378 binds to the mitochondrial transcriptome locus of ATP6, which is a subunit of the F0 complex of the complex V (ATP synthase) and finally impacts ATP generation [145]. During the process of skeletal muscle maturation, miR-1/133a targets the Mef2A/Dik1-Dio3 gene cluster and modulates the expression of multiple miRNAs which then suppress the mitochondrial genes [146].
Conformation of the existence of mitomiRs in the kidney tissue and exploration of their pathophysiologic functions will be of great interest and promising.
Canonical miRNA and mitochondria
It is shown that a couple of canonical miRNAs regulates mitochondrial functions including TCA, OXPHOS via mechanisms in the cytosol. Brain-specific miRNA, miR-338 reduces nuclear genome encoded cytochrome c oxidase subunit IV (COX4), which regulates ROS level [147]. Under hypoxic conditions, miR-210 is markedly induced and directly represses OXPHOS by targeting the iron-sulfur cluster scaffold (ISCU) and cytochrome c oxidase assembly protein (COX10), which ultimately contributes to the metabolic shift from OXPHOS to glycolysis [148, 149]. Moreover, miR-210 could regulate complex II activity by targeting its subunit succinate dehydrogenase subunit D (SDHD) [150]. miR-335 and miR-34a target mitochondrial superoxide dismutase 2 (SOD2) and thioredoxin reductase 2 (TR2) and therefore regulate oxidative damage and cell senescence [151]. Increased NADPH oxidase resulted from the decrease of miR-25 in diabetic kidney causes oxidative stress in mesenchymal cells [152].
The enzyme activity of pyruvate dehydrogenase (PDH) is reduced when its subunit X is targeted by miR-26a, which leads to accumulation of pyruvate with decrease of acetyl-coA [89]. It has been reported that citrate synthase (CS) is targeted by several miRNAs, including miR-152, −148a, −148b, − 299, −19a, −19b, −122a, − 421 and − 494 [153].
miR-124 downregulates succinate coA ligase GDP forming β subunit (SUCLG2) and represses the conversion of succinate to succinyl coA [154]. Downregulation of isocitrate dehydrogenase (IDH) by miR-183 and malate dehtdrogenase (MDH) by miR-743a within the TCA cycle results in a metabolic shift toward glycolytic status [155]. The ADP-ribosylation factor-like 2 (ARL2) is a common target for miR-15b, − 16, − 195, − 424 [156], which affects mitochondrial degradation and ATP production [157].
Other miRNAs have been implicated in modulation of mitochondrial dynamics. miR-30 family member are found to regulate Drp1 by targeting p53 [158]. Notably, miR-30/p53/Drp1 limits mitochondrial fission and promotes mitochondrial fusion, which has been suggested to be particularly important in high energy demanding organs such as the cardiac tissue [158]. miR-30/p53/Drp1 axis may also prevent the loss of cells with less self-renewal capacity by the increase of threshold for apoptotic activation [158]. This might be identified in kidney tissues that have the similar physiologic features.
miR-26 promotes mitochondrial uncoupling and induces energy dissipation in brown adipocytes by increasing uncoupling protein 1 (UCP1) and leads to a slight increase of cristae density [159]. Additionally, miR-27a and miR-27b were shown to regulate mitochondrial biogenesis, structure integrity and complex I activity during adipogenesis by targeting prohibitin [160]. The miR-149/poly (ADP-ribose) polymerase-1 (PARP-1)/NAD+/SIRT-1 axis increases mitochondrial function and biogenesis through PGC-1α activation in skeletal muscle [161].
miR-378 downregulates caspase 3 and inhibits apoptosis in cardiac tissue [162]. The aforementioned miR-1 targets insulin-like growth factor (IGF), decreases mitochondrial membrane potential and leads to the release of caspase 3 [163].
In summary, increasing evidences suggest that these mitochondrial functional regulating miRNAs are possibly mitomiRs and mediate nuclear regulation of mitochondrial functions and mitochondrial retrograde cellular adaptive signals (Fig. 5).
miRNA regulation of mitochondrial function and homeostasis. A schematic of miRNA-regulatory network in mitochondrial function and homeostasis. ARL2; ADP-ribosylation factor-like 2; COX, cytochrome c oxidase; CS, citrate synthase; IDH, isocitrate dehydrogenase; δψm, mitochondrial membrane potential; IGF, insulin-like growth factor; ISCU, iron-sulfur cluster scaffold; MDH, malate dehtdrogenase; mtDNA, mitochondrial DNA; OXPHOS, oxidative phosphorylation; PARP-1, poly (ADP-ribose) polymerase-1; SDH, succinate dehydrogenase; SOD2, superoxide dismutase 2; SUCLG2, succinate coA ligase GDP forming β subunit; TCA, tricarboxylic acid; TR2, thioredoxin reductase 2
Conclusion and perspective
Thousands of miRNAs have been shown to regulate numerous aspects in human physiological and pathological conditions. As we mentioned here, a growing number of miRNAs have been implicated in regulating metabolic disorders and maintaining mitochondrial homeostasis (Table 2). This could suggest similar regulatory roles of miRNAs in kidney metabolic diseases. It is necessary to carry out functional validation studies in human and models of kidney diseases to establish such link between miRNA expressions and their regulatory role in renal metabolic disorders. Moreover, as compared to traditional medications toward several druggable targets, the potential therapeutic implications for treatment of kidney diseases by targeting the aberrant miRNAs seem exciting in the clinical perspective. However, proteins are probably regulated by plenty of miRNAs because of the multiple target sites in mRNAs. In addition, miRNAs always have many target proteins because of the similar target sequences in mRNAs. The possible off-target effect and long-term consequences of miRNA-targeted therapeutics remain unknown. These will certainly be the topics for intensive research in the near future.
Availability of data and materials
Not applicable.
Abbreviations
- ACC1:
-
Acetyl-coA carboxylase
- ACSL:
-
Acyl-coA synthetase long chain
- ADPKD:
-
Autosomal dominant polycystic kidney disease
- Ago2:
-
Argonaut proteins
- AKI:
-
Acute kidney injury
- AMPK:
-
AMP-dependent kinase
- ARL2:
-
ADP-ribosylation factor-like 2
- CAV:
-
Caveolin
- COX:
-
Cytochrome c oxidase
- CPT1α:
-
Carnitine palmitoyltransferase 1α
- CROT:
-
Carnitine ctanoyl transferase
- CS:
-
Citrate synthase
- EGFR:
-
Epidermal growth factor receptor
- FASN:
-
Fatty acid synthase
- FFA:
-
Free fatty acid
- GAPDH:
-
Glyceraldehyde-3-phosphate dehydrogenase
- GLUT:
-
Glucose transporter
- HK2:
-
Hexokinase 2
- hnRNPA:
-
Hetergenous nucler ribonucleoprotein A
- IDH:
-
Isocitrate dehydrogenase
- IGF:
-
Insulin-like growth factor
- IGF1R:
-
Insulin-like growth factor 1 receptor
- INSIG1:
-
Insulin-induced gene 1
- IRS:
-
Insulin receptor substrate
- ISCU:
-
Iron-sulfur cluster scaffold
- LDH:
-
Lactate dehydrogenase
- MCT1:
-
Monocarboxylate transporter 1
- MDH:
-
Malate dehtdrogenase
- miRNA:
-
MicroRNA
- mtDNA:
-
Mitochondrial DNA
- ORP8:
-
Oxysterol-binding protein-related 8
- OXPHOS:
-
Oxidative phosphorylation
- PANK:
-
Pantothenate kinase
- PARP-1:
-
Poly (ADP-ribose) polymerase-1
- PDGFRA:
-
Platelet-derived growth factor receptor α
- PDH:
-
Pyruvate dehydrogenase
- PDHX:
-
Pyruvate dehydrogenase protein X
- PFK:
-
Phosphofructokinase
- PGK:
-
Phosphoglycerate kinase
- PKM2:
-
Pyruvate kinase type M2
- PPAR:
-
Peroxisome proliferator activated receptor
- PTCs:
-
Proximal tubular cells
- RISC:
-
RNA-induced silencing complex
- RTKs:
-
Receptor tyrosine kinases
- SDH:
-
Succinate dehydrogenase
- SHMT2:
-
Serine hydroxyl transferase
- shRNAs:
-
Short hairpin RNAs
- SOD2:
-
Superoxide dismutase 2
- SREBP:
-
Sterol-regulatory element binding proteins
- SUCLG2:
-
Succinate coA ligase GDP forming β subunit
- TAL:
-
Thick ascending loop of Henle
- TCA:
-
Tricarboxylic acid
- TR2:
-
Thioredoxin reductase 2
References
Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–97.
Yang JS, Lai EC. Alternative miRNA biogenesis pathways and the interpretation of core miRNA pathway mutants. Mol Cell. 2011;43(6):892–903.
Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136(2):215–33.
Mandel LJ, Balaban RS. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Phys. 1981;240(5):F357–71.
Soltoff SP. ATP and the regulation of renal cell function. Annu Rev Physiol. 1986;48:9–31.
Thaysen JH, Lassen NA, Munck O. Sodium transport and oxygen consumption in the mammalian kidney. Nature. 1961;190:919–21.
Wang Z, Ying Z, Bosy-Westphal A, Zhang J, Schautz B, Later W, Heymsfield SB, Muller MJ. Specific metabolic rates of major organs and tissues across adulthood: evaluation by mechanistic model of resting energy expenditure. Am J Clin Nutr. 2010;92(6):1369–77.
Pagliarini DJ, Calvo SE, Chang B, Sheth SA, Vafai SB, Ong SE, Walford GA, Sugiana C, Boneh A, Chen WK, et al. A mitochondrial protein compendium elucidates complex I disease biology. Cell. 2008;134(1):112–23.
O'Connor PM. Renal oxygen delivery: matching delivery to metabolic demand. Clin Exp Pharmacol Physiol. 2006;33(10):961–7.
Doucet A, Katz AI, Morel F. Determination of Na-K-ATPase activity in single segments of the mammalian nephron. Am J Phys. 1979;237(2):F105–13.
Katz AI, Doucet A, Morel F. Na-K-ATPase activity along the rabbit, rat, and mouse nephron. Am J Phys. 1979;237(2):F114–20.
Pfaller W, Rittinger M. Quantitative morphology of the rat kidney. Int J BioChemiPhysics. 1980;12(1–2):17–22.
Hall AM, Unwin RJ, Parker N, Duchen MR. Multiphoton imaging reveals differences in mitochondrial function between nephron segments. J Am Soc Nephrol. 2009;20(6):1293–302.
Katz AI. Distribution and function of classes of ATPases along the nephron. Kidney Int. 1986;29(1):21–31.
Mandel LJ. Metabolic substrates, cellular energy production, and the regulation of proximal tubular transport. Annu Rev Physiol. 1985;47:85–101.
Forbes JM, Thorburn DR. Mitochondrial dysfunction in diabetic kidney disease. Nat Rev Nephrol. 2018;14(5):291–312.
Rich PR. The molecular machinery of Keilin's respiratory chain. Biochem Soc Trans. 2003;31(Pt 6):1095–105.
Perriello G, Nurjhan N, Stumvoll M, Bucci A, Welle S, Dailey G, Bier DM, Toft I, Jenssen TG, Gerich JE. Regulation of gluconeogenesis by glutamine in normal postabsorptive humans. Am J Phys. 1997;272(3 Pt 1):E437–45.
Meyer C, Stumvoll M, Dostou J, Welle S, Haymond M, Gerich J. Renal substrate exchange and gluconeogenesis in normal postabsorptive humans. Am J Physiol Endocrinol Metab. 2002;282(2):E428–34.
Krebs HA, Yoshida T. Renal gluconeogenesis. 2. The Gluconeogenic capacity of the kidney cortex of various species. Biochem J. 1963;89:398–400.
Mc CW, Jude JR. The synthesis of glucose by the kidney. Bull Johns Hopkins Hosp. 1958;103(2):77–93.
Thomas SR. Inner medullary lactate production and accumulation: a vasa recta model. Am J Physiol Renal Physiol. 2000;279(3):F468–81.
Gerich JE. Role of the kidney in normal glucose homeostasis and in the hyperglycaemia of diabetes mellitus: therapeutic implications. Diabet Med. 2010;27(2):136–42.
Ross BD, Espinal J, Silva P. Glucose metabolism in renal tubular function. Kidney Int. 1986;29(1):54–67.
Chen Y, Fry BC, Layton AT. Modeling glucose metabolism and lactate production in the kidney. Math Biosci. 2017;289:116–29.
Guder WG, Ross BD. Enzyme distribution along the nephron. Kidney Int. 1984;26(2):101–11.
Wirthensohn G, Guder WG. Renal substrate metabolism. Physiol Rev. 1986;66(2):469–97.
Rodrigues B, Cam MC, McNeill JH. Metabolic disturbances in diabetic cardiomyopathy. Mol Cell Biochem. 1998;180(1–2):53–7.
Bonnefont JP, Djouadi F, Prip-Buus C, Gobin S, Munnich A, Bastin J. Carnitine palmitoyltransferases 1 and 2: biochemical, molecular and medical aspects. Mol Asp Med. 2004;25(5–6):495–520.
Goedeke L, Vales-Lara FM, Fenstermaker M, Cirera-Salinas D, Chamorro-Jorganes A, Ramirez CM, Mattison JA, de Cabo R, Suarez Y, Fernandez-Hernando C. A regulatory role for microRNA 33* in controlling lipid metabolism gene expression. Mol Cell Biol. 2013;33(11):2339–52.
Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nat Rev Mol Cell Biol. 2012;13(4):239–50.
Iliopoulos D, Drosatos K, Hiyama Y, Goldberg IJ, Zannis VI. MicroRNA-370 controls the expression of microRNA-122 and Cpt1alpha and affects lipid metabolism. J Lipid Res. 2010;51(6):1513–23.
Sun Y, Oravecz-Wilson K, Bridges S, McEachin R, Wu J, Kim SH, Taylor A, Zajac C, Fujiwara H. Peltier DC et al: miR-142 controls metabolic reprogramming that regulates dendritic cell activation. J Clin Invest. 2019;130:2029–42.
Gerin I, Clerbaux LA, Haumont O, Lanthier N, Das AK, Burant CF, Leclercq IA, MacDougald OA, Bommer GT. Expression of miR-33 from an SREBP2 intron inhibits cholesterol export and fatty acid oxidation. J Biol Chem. 2010;285(44):33652–61.
Bommer GT, MacDougald OA. Regulation of lipid homeostasis by the bifunctional SREBF2-miR33a locus. Cell Metab. 2011;13(3):241–7.
Xu X, So JS, Park JG, Lee AH. Transcriptional control of hepatic lipid metabolism by SREBP and ChREBP. Semin Liver Dis. 2013;33(4):301–11.
Davalos A, Goedeke L, Smibert P, Ramirez CM, Warrier NP, Andreo U, Cirera-Salinas D, Rayner K, Suresh U, Pastor-Pareja JC, et al. miR-33a/b contribute to the regulation of fatty acid metabolism and insulin signaling. Proc Natl Acad Sci U S A. 2011;108(22):9232–7.
Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. Genomic instability and aging-like phenotype in the absence of mammalian SIRT6. Cell. 2006;124(2):315–29.
Zhong L, D'Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, et al. The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1alpha. Cell. 2010;140(2):280–93.
Xiao C, Kim HS, Lahusen T, Wang RH, Xu X, Gavrilova O, Jou W, Gius D, Deng CX. SIRT6 deficiency results in severe hypoglycemia by enhancing both basal and insulin-stimulated glucose uptake in mice. J Biol Chem. 2010;285(47):36776–84.
Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, et al. SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 2008;452(7186):492–6.
Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, R MK, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006;3(2):87–98.
Krutzfeldt J, Rajewsky N, Braich R, Rajeev KG, Tuschl T, Manoharan M, Stoffel M. Silencing of microRNAs in vivo with 'antagomirs'. Nature. 2005;438(7068):685–9.
Tomasiak TM, Cecchini G, Iverson TM. Succinate as donor; Fumarate as acceptor. EcoSal Plus. 2007;2(2). https://doi.org/10.1128/ecosal.3.2.6.
Wilfred BR, Wang WX, Nelson PT. Energizing miRNA research: a review of the role of miRNAs in lipid metabolism, with a prediction that miR-103/107 regulates human metabolic pathways. Mol Genet Metab. 2007;91(3):209–17.
Peng Y, Xiang H, Chen C, Zheng R, Chai J, Peng J, Jiang S. MiR-224 impairs adipocyte early differentiation and regulates fatty acid metabolism. Int J Biochem Cell Biol. 2013;45(8):1585–93.
Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, Li J, Tran PT, Kaimal V, Huang X, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4(121):121ra118.
Gomez IG, MacKenna DA, Johnson BG, Kaimal V, Roach AM, Ren S, Nakagawa N, Xin C, Newitt R, Pandya S, et al. Anti-microRNA-21 oligonucleotides prevent Alport nephropathy progression by stimulating metabolic pathways. J Clin Invest. 2015;125(1):141–56.
Hajarnis S, Lakhia R, Yheskel M, Williams D, Sorourian M, Liu X, Aboudehen K, Zhang S, Kersjes K, R G, et al. microRNA-17 family promotes polycystic kidney disease progression through modulation of mitochondrial metabolism. Nat Commun. 2017;8:14395.
Yan W, Wu X, Zhou W, Fong MY, Cao M, Liu J, Liu X, Chen CH, Fadare O, Pizzo DP, et al. Cancer-cell-secreted exosomal miR-105 promotes tumour growth through the MYC-dependent metabolic reprogramming of stromal cells. Nat Cell Biol. 2018;20(5):597–609.
Burkart EM, Sambandam N, Han X, Gross RW, Courtois M, Gierasch CM, Shoghi K, Welch MJ, Kelly DP. Nuclear receptors PPARbeta/delta and PPARalpha direct distinct metabolic regulatory programs in the mouse heart. J Clin Invest. 2007;117(12):3930–9.
el Azzouzi H, Leptidis S, Dirkx E, Hoeks J, van Bree B, Brand K, McClellan EA, Poels E, Sluimer JC, van den Hoogenhof MM, et al. The hypoxia-inducible microRNA cluster miR-199a approximately 214 targets myocardial PPARdelta and impairs mitochondrial fatty acid oxidation. Cell Metab. 2013;18(3):341–54.
Kurtz CL, Peck BC, Fannin EE, Beysen C, Miao J, Landstreet SR, Ding S, Turaga V, Lund PK, Turner S, et al. MicroRNA-29 fine-tunes the expression of key FOXA2-activated lipid metabolism genes and is dysregulated in animal models of insulin resistance and diabetes. Diabetes. 2014;63(9):3141–8.
Hardie DG. AMP-activated protein kinase: a cellular energy sensor with a key role in metabolic disorders and in cancer. Biochem Soc Trans. 2011;39(1):1–13.
Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;478(7369):404–7.
Li Y, Xu S, Mihaylova MM, Zheng B, Hou X, Jiang B, Park O, Luo Z, Lefai E, Shyy JY, et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 2011;13(4):376–88.
Yap F, Craddock L, Yang J. Mechanism of AMPK suppression of LXR-dependent Srebp-1c transcription. Int J Biol Sci. 2011;7(5):645–50.
Kohjima M, Higuchi N, Kato M, Kotoh K, Yoshimoto T, Fujino T, Yada M, Yada R, Harada N, Enjoji M, et al. SREBP-1c, regulated by the insulin and AMPK signaling pathways, plays a role in nonalcoholic fatty liver disease. Int J Mol Med. 2008;21(4):507–11.
He A, Zhu L, Gupta N, Chang Y, Fang F. Overexpression of micro ribonucleic acid 29, highly up-regulated in diabetic rats, leads to insulin resistance in 3T3-L1 adipocytes. Mol Endocrinol. 2007;21(11):2785–94.
Kim S, Pak Y. Caveolin-2 regulation of the cell cycle in response to insulin in Hirc-B fibroblast cells. Biochem Biophys Res Commun. 2005;330(1):88–96.
Pandey AK, Verma G, Vig S, Srivastava S, Srivastava AK. Datta M: miR-29a levels are elevated in the db/db mice liver and its overexpression leads to attenuation of insulin action on PEPCK gene expression in HepG2 cells. Mol Cell Endocrinol. 2011;332(1–2):125–33.
Ryu HS, Park SY, Ma D, Zhang J, Lee W. The induction of microRNA targeting IRS-1 is involved in the development of insulin resistance under conditions of mitochondrial dysfunction in hepatocytes. PLoS One. 2011;6(3):e17343.
Lu H, Buchan RJ, Cook SA. MicroRNA-223 regulates Glut4 expression and cardiomyocyte glucose metabolism. Cardiovasc Res. 2010;86(3):410–20.
Naar AM. MiRs with a sweet tooth. Cell Metab. 2011;14(2):149–50.
Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;474(7353):649–53.
Martello G, Rosato A, Ferrari F, Manfrin A, Cordenonsi M, Dupont S, Enzo E, Guzzardo V, Rondina M, Spruce T, et al. A MicroRNA targeting dicer for metastasis control. Cell. 2010;141(7):1195–207.
Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Bottger T, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat Cell Biol. 2011;13(4):434–46.
Zhu H, Shyh-Chang N, Segre AV, Shinoda G, Shah SP, Einhorn WS, Takeuchi A, Engreitz JM, Hagan JP, Kharas MG, et al. The Lin28/let-7 axis regulates glucose metabolism. Cell. 2011;147(1):81–94.
Dai DW, Lu Q, Wang LX, Zhao WY, Cao YQ, Li YN, Han GS, Liu JM, Yue ZJ. Decreased miR-106a inhibits glioma cell glucose uptake and proliferation by targeting SLC2A3 in GBM. BMC Cancer. 2013;13:478.
Liu Y, Li YM, Tian RF, Liu WP, Fei Z, Long QF, Wang XA, Zhang X. The expression and significance of HIF-1alpha and GLUT-3 in glioma. Brain Res. 2009;1304:149–54.
Fei X, Qi M, Wu B, Song Y, Wang Y, Li T. MicroRNA-195-5p suppresses glucose uptake and proliferation of human bladder cancer T24 cells by regulating GLUT3 expression. FEBS Lett. 2012;586(4):392–7.
Liu M, Gao J, Huang Q, Jin Y, Wei Z. Downregulating microRNA-144 mediates a metabolic shift in lung cancer cells by regulating GLUT1 expression. Oncol Lett. 2016;11(6):3772–6.
Yamasaki T, Seki N, Yoshino H, Itesako T, Yamada Y, Tatarano S, Hidaka H, Yonezawa T, Nakagawa M, Enokida H. Tumor-suppressive microRNA-1291 directly regulates glucose transporter 1 in renal cell carcinoma. Cancer Sci. 2013;104(11):1411–9.
Santasusagna S, Moreno I, Navarro A, Munoz C, Martinez F, Hernandez R, Castellano JJ. Monzo M: miR-328 mediates a metabolic shift in colon cancer cells by targeting SLC2A1/GLUT1. Clin Transl Oncol. 2018;20(9):1161–7.
Zhao S, Liu H, Liu Y, Wu J, Wang C, Hou X, Chen X, Yang G, Zhao L, H C, et al. miR-143 inhibits glycolysis and depletes stemness of glioblastoma stem-like cells. Cancer Lett. 2013;333(2):253–60.
Jiang S, Zhang LF, Zhang HW, Hu S, Lu MH, Liang S, Li B, Li Y, Li D, Wang ED, et al. A novel miR-155/miR-143 cascade controls glycolysis by regulating hexokinase 2 in breast cancer cells. EMBO J. 2012;31(8):1985–98.
Li W, Huang H, Su J, Ji X, Zhang X, Zhang Z. Wang H: miR-124 acts as a tumor suppressor in Glioblastoma via the inhibition of signal transducer and activator of transcription 3. Mol Neurobiol. 2017;54(4):2555–61.
Yang J, Li J, Le Y, Zhou C, Zhang S, Gong Z. PFKL/miR-128 axis regulates glycolysis by inhibiting AKT phosphorylation and predicts poor survival in lung cancer. Am J Cancer Res. 2016;6(2):473–85.
Tang H, Lee M, Sharpe O, Salamone L, Noonan EJ, Hoang CD, Levine S, Robinson WH, Shrager JB. Oxidative stress-responsive microRNA-320 regulates glycolysis in diverse biological systems. FASEB J. 2012;26(11):4710–21.
Yang Y, Ishak Gabra MB, Hanse EA, Lowman XH, Tran TQ, Li H, Milman N, Liu J, Reid MA, Locasale JW, et al. MiR-135 suppresses glycolysis and promotes pancreatic cancer cell adaptation to metabolic stress by targeting phosphofructokinase-1. Nat Commun. 2019;10(1):809.
Cha Y, Han MJ, Cha HJ, Zoldan J, Burkart A, Jung JH, Jang Y, Kim CH, Jeong HC, Kim BG, et al. Metabolic control of primed human pluripotent stem cell fate and function by the miR-200c-SIRT2 axis. Nat Cell Biol. 2017;19(5):445–56.
Luan W, Wang Y, Chen X, Shi Y, Wang J, Zhang J, Qian J, Li R, Tao T, Wei W, et al. PKM2 promotes glucose metabolism and cell growth in gliomas through a mechanism involving a let-7a/c-Myc/hnRNPA1 feedback loop. Oncotarget. 2015;6(15):13006–18.
Kefas B, Comeau L, Erdle N, Montgomery E, Amos S, Purow B. Pyruvate kinase M2 is a target of the tumor-suppressive microRNA-326 and regulates the survival of glioma cells. Neuro-Oncology. 2010;12(11):1102–12.
Sakr M, Takino T, Sabit H, Nakada M, Li Z. Sato H: miR-150-5p and miR-133a suppress glioma cell proliferation and migration through targeting membrane-type-1 matrix metalloproteinase. Gene. 2016;587(2):155–62.
Wong TS, Liu XB, Chung-Wai Ho A, Po-Wing Yuen A, Wai-Man Ng R, Ignace Wei W. Identification of pyruvate kinase type M2 as potential oncoprotein in squamous cell carcinoma of tongue through microRNA profiling. Int J Cancer. 2008;123(2):251–7.
Chang L, Lei X, Qin YU, Zhang X, Jin H, Wang C, Wang X, Li G, Tan C, Su J. MicroRNA-133b inhibits cell migration and invasion by targeting matrix metalloproteinase 14 in glioblastoma. Oncol Lett. 2015;10(5):2781–6.
Liu AM, Xu Z, Shek FH, Wong KF, Lee NP, Poon RT, Chen J. Luk JM: miR-122 targets pyruvate kinase M2 and affects metabolism of hepatocellular carcinoma. PLoS One. 2014;9(1):e86872.
Sun Y, Zhao X, Zhou Y. Hu Y: miR-124, miR-137 and miR-340 regulate colorectal cancer growth via inhibition of the Warburg effect. Oncol Rep. 2012;28(4):1346–52.
Chen B, Liu Y, Jin X, Lu W, Liu J, Xia Z, Yuan Q, Zhao X, Xu N, Liang S. MicroRNA-26a regulates glucose metabolism by direct targeting PDHX in colorectal cancer cells. BMC Cancer. 2014;14:443.
Peurala H, Greco D, Heikkinen T, Kaur S, Bartkova J, Jamshidi M, Aittomaki K, Heikkila P, Bartek J, Blomqvist C, et al. MiR-34a expression has an effect for lower risk of metastasis and associates with expression patterns predicting clinical outcome in breast cancer. PLoS One. 2011;6(11):e26122.
Xiao X, Huang X, Ye F, Chen B, Song C, Wen J, Zhang Z, Zheng G, Tang H, Xie X. The miR-34a-LDHA axis regulates glucose metabolism and tumor growth in breast cancer. Sci Rep. 2016;6:21735.
Isozaki Y, Hoshino I, Nohata N, Kinoshita T, Akutsu Y, Hanari N, Mori M, Yoneyama Y, Akanuma N, Takeshita N, et al. Identification of novel molecular targets regulated by tumor suppressive miR-375 induced by histone acetylation in esophageal squamous cell carcinoma. Int J Oncol. 2012;41(3):985–94.
Kinoshita T, Nohata N, Yoshino H, Hanazawa T, Kikkawa N, Fujimura L, Chiyomaru T, Kawakami K, Enokida H, Nakagawa M, et al. Tumor suppressive microRNA-375 regulates lactate dehydrogenase B in maxillary sinus squamous cell carcinoma. Int J Oncol. 2012;40(1):185–93.
Chang C, Shi H, Wang C, Wang J, Geng N, Jiang X, Wang X. Correlation of microRNA-375 downregulation with unfavorable clinical outcome of patients with glioma. Neurosci Lett. 2012;531(2):204–8.
Li KK, Pang JC, Ching AK, Wong CK, Kong X, Wang Y, Zhou L, Chen Z. Ng HK: miR-124 is frequently down-regulated in medulloblastoma and is a negative regulator of SLC16A1. Hum Pathol. 2009;40(9):1234–43.
Romero-Cordoba SL, Rodriguez-Cuevas S, Bautista-Pina V, Maffuz-Aziz A, D'Ippolito E, Cosentino G, Baroni S, Iorio MV, Hidalgo-Miranda A. Loss of function of miR-342-3p results in MCT1 over-expression and contributes to oncogenic metabolic reprogramming in triple negative breast cancer. Sci Rep. 2018;8(1):12252.
Chen L, Zhang J, Feng Y, Li R, Sun X, Du W, Piao X, Wang H, Yang D, Sun Y, et al. MiR-410 regulates MET to influence the proliferation and invasion of glioma. Int J Biochem Cell Biol. 2012;44(11):1711–7.
Lan F, Yu H, Hu M, Xia T. Yue X: miR-144-3p exerts anti-tumor effects in glioblastoma by targeting c-met. J Neurochem. 2015;135(2):274–86.
Luan S, Sun L, Huang F. MicroRNA-34a: a novel tumor suppressor in p53-mutant glioma cell line U251. Arch Med Res. 2010;41(2):67–74.
Li Y, Guessous F, Zhang Y, Dipierro C, Kefas B, Johnson E, Marcinkiewicz L, Jiang J, Yang Y, Schmittgen TD, et al. MicroRNA-34a inhibits glioblastoma growth by targeting multiple oncogenes. Cancer Res. 2009;69(19):7569–76.
Rathod SS, Rani SB, Khan M, Muzumdar D, Shiras A. Tumor suppressive miRNA-34a suppresses cell proliferation and tumor growth of glioma stem cells by targeting Akt and Wnt signaling pathways. FEBS Open Bio. 2014;4:485–95.
Silber J, Jacobsen A, Ozawa T, Harinath G, Pedraza A, Sander C, Holland EC. Huse JT: miR-34a repression in proneural malignant gliomas upregulates expression of its target PDGFRA and promotes tumorigenesis. PLoS One. 2012;7(3):e33844.
Papagiannakopoulos T, Friedmann-Morvinski D, Neveu P, Dugas JC, Gill RM, Huillard E, Liu C, Zong H, Rowitch DH, Barres BA, et al. Pro-neural miR-128 is a glioma tumor suppressor that targets mitogenic kinases. Oncogene. 2012;31(15):1884–95.
Rao SA, Santosh V, Somasundaram K. Genome-wide expression profiling identifies deregulated miRNAs in malignant astrocytoma. Mod Pathol. 2010;23(10):1404–17.
Rao SA, Arimappamagan A, Pandey P, Santosh V, Hegde AS, Chandramouli BA. Somasundaram K: miR-219-5p inhibits receptor tyrosine kinase pathway by targeting EGFR in glioblastoma. PLoS One. 2013;8(5):e63164.
Kefas B, Godlewski J, Comeau L, Li Y, Abounader R, Hawkinson M, Lee J, Fine H, Chiocca EA, S L, et al. microRNA-7 inhibits the epidermal growth factor receptor and the Akt pathway and is down-regulated in glioblastoma. Cancer Res. 2008;68(10):3566–72.
Webster RJ, Giles KM, Price KJ, Zhang PM, Mattick JS, Leedman PJ. Regulation of epidermal growth factor receptor signaling in human cancer cells by microRNA-7. J Biol Chem. 2009;284(9):5731–41.
Tan X, Wang S, Yang B, Zhu L, Yin B, Chao T, Zhao J, Yuan J, Qiang B, Peng X. The CREB-miR-9 negative feedback minicircuitry coordinates the migration and proliferation of glioma cells. PLoS One. 2012;7(11):e49570.
Wang L, Shi ZM, Jiang CF, Liu X, Chen QD, Qian X, Li DM, Ge X, Wang XF, Liu LZ, et al. MiR-143 acts as a tumor suppressor by targeting N-RAS and enhances temozolomide-induced apoptosis in glioma. Oncotarget. 2014;5(14):5416–27.
Fiore D, Donnarumma E, Roscigno G, Iaboni M, Russo V, Affinito A, Adamo A, De Martino F, Quintavalle C, G R, et al. miR-340 predicts glioblastoma survival and modulates key cancer hallmarks through down-regulation of NRAS. Oncotarget. 2016;7(15):19531–47.
Huang D, Qiu S, Ge R, He L, Li M, Li Y. Peng Y: miR-340 suppresses glioblastoma multiforme. Oncotarget. 2015;6(11):9257–70.
Kim H, Huang W, Jiang X, Pennicooke B, Park PJ, Johnson MD. Integrative genome analysis reveals an oncomir/oncogene cluster regulating glioblastoma survivorship. Proc Natl Acad Sci U S A. 2010;107(5):2183–8.
Zhang Y, Kim J, Mueller AC, Dey B, Yang Y, Lee DH, Hachmann J, Finderle S, Park DM, Christensen J, et al. Multiple receptor tyrosine kinases converge on microRNA-134 to control KRAS, STAT5B, and glioblastoma. Cell Death Differ. 2014;21(5):720–34.
Liu Z, Jiang Z, Huang J, Huang S, Li Y, Yu S. Liu X: miR-7 inhibits glioblastoma growth by simultaneously interfering with the PI3K/ATK and Raf/MEK/ERK pathways. Int J Oncol. 2014;44(5):1571–80.
Cai J, Zhao J, Zhang N, Xu X, Li R, Yi Y, Fang L, Zhang L, Li M, Wu J, et al. MicroRNA-542-3p suppresses tumor cell invasion via targeting AKT pathway in human astrocytoma. J Biol Chem. 2015;290(41):24678–88.
Zhou X, Ren Y, Moore L, Mei M, You Y, Xu P, Wang B, Wang G, Jia Z, Pu P, et al. Downregulation of miR-21 inhibits EGFR pathway and suppresses the growth of human glioblastoma cells independent of PTEN status. Lab Investig. 2010;90(2):144–55.
Guo P, Nie Q, Lan J, Ge J, Qiu Y, Mao Q. C-Myc negatively controls the tumor suppressor PTEN by upregulating miR-26a in glioblastoma multiforme cells. Biochem Biophys Res Commun. 2013;441(1):186–90.
Xia X, Li Y, Wang W, Tang F, Tan J, Sun L, Li Q, Tang B, He S. MicroRNA-1908 functions as a glioblastoma oncogene by suppressing PTEN tumor suppressor pathway. Mol Cancer. 2015;14:154.
Li XT, Wang HZ, Wu ZW, Yang TQ, Zhao ZH, Chen GL, Xie XS, Li B, Wei YX, Huang YL, et al. miR-494-3p regulates cellular proliferation, invasion, migration, and apoptosis by PTEN/AKT signaling in human Glioblastoma cells. Cell Mol Neurobiol. 2015;35(5):679–87.
Liu S, Sun J, Lan Q. TGF-beta-induced miR10a/b expression promotes human glioma cell migration by targeting PTEN. Mol Med Rep. 2013;8(6):1741–6.
Ciafre SA, Galardi S, Mangiola A, Ferracin M, Liu CG, Sabatino G, Negrini M, Maira G, Croce CM, Farace MG. Extensive modulation of a set of microRNAs in primary glioblastoma. Biochem Biophys Res Commun. 2005;334(4):1351–8.
Conti A, Aguennouz M, La Torre D, Tomasello C, Cardali S, Angileri FF, Maio F, Cama A, Germano A, G V, et al. miR-21 and 221 upregulation and miR-181b downregulation in human grade II-IV astrocytic tumors. J Neuro-Oncol. 2009;93(3):325–32.
Luo Z, Zang M, Guo W. AMPK as a metabolic tumor suppressor: control of metabolism and cell growth. Future Oncol. 2010;6(3):457–70.
Godlewski J, Nowicki MO, Bronisz A, Nuovo G, Palatini J, De Lay M, Van Brocklyn J, Ostrowski MC, Chiocca EA, Lawler SE. MicroRNA-451 regulates LKB1/AMPK signaling and allows adaptation to metabolic stress in glioma cells. Mol Cell. 2010;37(5):620–32.
Shen L, Sun C, Li Y, Li X, Sun T, Liu C, Zhou Y, Du Z. MicroRNA-199a-3p suppresses glioma cell proliferation by regulating the AKT/mTOR signaling pathway. Tumour Biol. 2015;36(9):6929–38.
Hresko RC, Mueckler M. mTOR.RICTOR is the Ser473 kinase for Akt/protein kinase B in 3T3-L1 adipocytes. J Biol Chem. 2005;280(49):40406–16.
Masui K, Tanaka K, Akhavan D, Babic I, Gini B, Matsutani T, Iwanami A, Liu F, Villa GR, Gu Y, et al. mTOR complex 2 controls glycolytic metabolism in glioblastoma through FoxO acetylation and upregulation of c-Myc. Cell Metab. 2013;18(5):726–39.
Ling N, Gu J, Lei Z, Li M, Zhao J, Zhang HT. Li X: microRNA-155 regulates cell proliferation and invasion by targeting FOXO3a in glioma. Oncol Rep. 2013;30(5):2111–8.
Leivonen SK, Rokka A, Ostling P, Kohonen P, Corthals GL, Kallioniemi O, Perala M. Identification of miR-193b targets in breast cancer cells and systems biological analysis of their functional impact. Mol Cell Proteomics. 2011;10(7):M110 005322.
Gao P, Tchernyshyov I, Chang TC, Lee YS, Kita K, Ochi T, Zeller KI, De Marzo AM, Van Eyk JE, Mendell JT, et al. C-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature. 2009;458(7239):762–5.
Mersey BD, Jin P, Danner DJ. Human microRNA (miR29b) expression controls the amount of branched chain alpha-ketoacid dehydrogenase complex in a cell. Hum Mol Genet. 2005;14(22):3371–7.
Bandiera S, Mategot R, Girard M, Demongeot J, Henrion-Caude A. MitomiRs delineating the intracellular localization of microRNAs at mitochondria. Free Radic Biol Med. 2013;64:12–9.
Barrey E, Saint-Auret G, Bonnamy B, Damas D, Boyer O, Gidrol X. Pre-microRNA and mature microRNA in human mitochondria. PLoS One. 2011;6(5):e20220.
Ro S, Ma HY, Park C, Ortogero N, Song R, Hennig GW, Zheng H, Lin YM, Moro L, Hsieh JT, et al. The mitochondrial genome encodes abundant small noncoding RNAs. Cell Res. 2013;23(6):759–74.
Zhang X, Zuo X, Yang B, Li Z, Xue Y, Zhou Y, Huang J, Zhao X, Zhou J, Yan Y, et al. MicroRNA directly enhances mitochondrial translation during muscle differentiation. Cell. 2014;158(3):607–19.
Bandiera S, Ruberg S, Girard M, Cagnard N, Hanein S, Chretien D, Munnich A, Lyonnet S, Henrion-Caude A. Nuclear outsourcing of RNA interference components to human mitochondria. PLoS One. 2011;6(6):e20746.
Yuan JD, Shi JX, Meng GX, An LG, Hu GX. Nuclear pseudogenes of mitochondrial DNA as a variable part of the human genome. Cell Res. 1999;9(4):281–90.
Tsuzuki T, Nomiyama H, Setoyama C, Maeda S, Shimada K. Presence of mitochondrial-DNA-like sequences in the human nuclear DNA. Gene. 1983;25(2–3):223–9.
Woischnik M, Moraes CT. Pattern of organization of human mitochondrial pseudogenes in the nuclear genome. Genome Res. 2002;12(6):885–93.
Das S, Ferlito M, Kent OA, Fox-Talbot K, Wang R, Liu D, Raghavachari N, Yang Y, Wheelan SJ, Murphy E, et al. Nuclear miRNA regulates the mitochondrial genome in the heart. Circ Res. 2012;110(12):1596–603.
Das S, Bedja D, Campbell N, Dunkerly B, Chenna V, Maitra A. Steenbergen C: miR-181c regulates the mitochondrial genome, bioenergetics, and propensity for heart failure in vivo. PLoS One. 2014;9(5):e96820.
Wang L, Huang H, Fan Y, Kong B, Hu H, Hu K, Guo J, Mei Y, Liu WL. Effects of downregulation of microRNA-181a on H2O2-induced H9c2 cell apoptosis via the mitochondrial apoptotic pathway. Oxidative Med Cell Longev. 2014;2014:960362.
Zhu HY, Liu MY, Hong Q, Zhang D, Geng WJ, Xie YS, Chen XM. Role of microRNA-181a in the apoptosis of tubular epithelial cell induced by cisplatin. Chin Med J. 2012;125(3):523–6.
Rippo MR, Olivieri F, Monsurro V, Prattichizzo F, Albertini MC, Procopio AD. MitomiRs in human inflamm-aging: a hypothesis involving miR-181a, miR-34a and miR-146a. Exp Gerontol. 2014;56:154–63.
Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Stricker JC, Croston TL, Baseler WA, Lewis SE, Martinez I, Hollander JM. Translational regulation of the mitochondrial genome following redistribution of mitochondrial MicroRNA in the diabetic heart. Circ Cardiovasc Genet. 2015;8(6):785–802.
Wust S, Drose S, Heidler J, Wittig I, Klockner I, Franko A, Bonke E, Gunther S, Gartner U, Boettger T, et al. Metabolic maturation during muscle stem cell differentiation is achieved by miR-1/133a-mediated inhibition of the Dlk1-Dio3 mega gene cluster. Cell Metab. 2018;27(5):1026–39 e1026.
Aschrafi A, Kar AN, Natera-Naranjo O, MacGibeny MA, Gioio AE, Kaplan BB. MicroRNA-338 regulates the axonal expression of multiple nuclear-encoded mitochondrial mRNAs encoding subunits of the oxidative phosphorylation machinery. Cell Mol Life Sci. 2012;69(23):4017–27.
Chan SY, Zhang YY, Hemann C, Mahoney CE, Zweier JL, Loscalzo J. MicroRNA-210 controls mitochondrial metabolism during hypoxia by repressing the iron-sulfur cluster assembly proteins ISCU1/2. Cell Metab. 2009;10(4):273–84.
Chen Z, Li Y, Zhang H, Huang P, Luthra R. Hypoxia-regulated microRNA-210 modulates mitochondrial function and decreases ISCU and COX10 expression. Oncogene. 2010;29(30):4362–8.
Puissegur MP, Mazure NM, Bertero T, Pradelli L, Grosso S, Robbe-Sermesant K, Maurin T, Lebrigand K, Cardinaud B. Hofman V et al: miR-210 is overexpressed in late stages of lung cancer and mediates mitochondrial alterations associated with modulation of HIF-1 activity. Cell Death Differ. 2011;18(3):465–78.
Bai XY, Ma Y, Ding R, Fu B, Shi S. Chen XM: miR-335 and miR-34a promote renal senescence by suppressing mitochondrial antioxidative enzymes. J Am Soc Nephrol. 2011;22(7):1252–61.
Fu Y, Zhang Y, Wang Z, Wang L, Wei X, Zhang B, Wen Z, Fang H, Pang Q, Yi F. Regulation of NADPH oxidase activity is associated with miRNA-25-mediated NOX4 expression in experimental diabetic nephropathy. Am J Nephrol. 2010;32(6):581–9.
Baradan R, Hollander JM, Das S. Mitochondrial miRNAs in diabetes: just the tip of the iceberg. Can J Physiol Pharmacol. 2017;95(10):1156–62.
Wang X. Systematic identification of microRNA functions by combining target prediction and expression profiling. Nucleic Acids Res. 2006;34(5):1646–52.
Bienertova-Vasku J, Sana J, Slaby O. The role of microRNAs in mitochondria in cancer. Cancer Lett. 2013;336(1):1–7.
Nishi H, Ono K, Iwanaga Y, Horie T, Nagao K, Takemura G, Kinoshita M, Kuwabara Y, Mori RT, Hasegawa K, et al. MicroRNA-15b modulates cellular ATP levels and degenerates mitochondria via Arl2 in neonatal rat cardiac myocytes. J Biol Chem. 2010;285(7):4920–30.
Tomasetti M, Neuzil J, Dong L. MicroRNAs as regulators of mitochondrial function: role in cancer suppression. Biochim Biophys Acta. 2014;1840(4):1441–53.
Li J, Donath S, Li Y, Qin D, Prabhakar BS. Li P: miR-30 regulates mitochondrial fission through targeting p53 and the dynamin-related protein-1 pathway. PLoS Genet. 2010;6(1):e1000795.
Karbiener M, Pisani DF, Frontini A, Oberreiter LM, Lang E, Vegiopoulos A, Mossenbock K, Bernhardt GA, Mayr T, Hildner F, et al. MicroRNA-26 family is required for human adipogenesis and drives characteristics of brown adipocytes. Stem Cells. 2014;32(6):1578–90.
Kang T, Lu W, Xu W, Anderson L, Bacanamwo M, Thompson W, Chen YE, Liu D. MicroRNA-27 (miR-27) targets prohibitin and impairs adipocyte differentiation and mitochondrial function in human adipose-derived stem cells. J Biol Chem. 2013;288(48):34394–402.
Mohamed JS, Hajira A, Pardo PS, Boriek AM. MicroRNA-149 inhibits PARP-2 and promotes mitochondrial biogenesis via SIRT-1/PGC-1alpha network in skeletal muscle. Diabetes. 2014;63(5):1546–59.
Fang J, Song XW, Tian J, Chen HY, Li DF, Wang JF, Ren AJ, Yuan WJ, Lin L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis. 2012;17(4):410–23.
Yu XY, Song YH, Geng YJ, Lin QX, Shan ZX, Lin SG, Li Y. Glucose induces apoptosis of cardiomyocytes via microRNA-1 and IGF-1. Biochem Biophys Res Commun. 2008;376(3):548–52.
Acknowledgements
Not applicable.
Funding
This work was supported by Key Program of National Natural Science Foundation of China 81530022, General Program of National Natural Science Foundation of China 81873618 and National Natural Science Foundation for Young Scholars of China 81600526.
Author information
Authors and Affiliations
Contributions
YZ was a major contributor in writing the manuscript. JY contributed in writing and revising the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Zhou, Y., Yang, J. Implications of microRNA in kidney metabolic disorders. ExRNA 2, 4 (2020). https://doi.org/10.1186/s41544-019-0042-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41544-019-0042-9