Abstract
Hypertension is a well-recognized risk factor for the development of cardiovascular disease, and the early detection of cardiac changes from hypertension can allow reversing these. Hypertensive heart diseases (HHD) refer to the complex and diverse change of the cardiac structure and function secondary to hypertension. Although conventional echocardiography is the most common imaging modality in detecting HHD, it cannot detect subtle changes of cardiac structure in subclinical states. Because strain echocardiography is another echocardiographic modality can detect subclinical myocardial dysfunction by measuring intrinsic myocardial deformation, it became more and more popular in clinical and research fields. In this review article, we described the basic concept of strain echocardiography and summarized several clinical studies showing its clinical utilities in the detection of HHD.
Similar content being viewed by others
Background
Hypertension is the most important cardiovascular risk factor with an increased risk of heart failure (HF), myocardial infarction, stroke, and cardiovascular death [1,2,3,4]. Hypertensive heart diseases (HHD, Fig. 1) include cardiac conditions caused by chronically elevated blood pressure, and these include HF, ischemic heart diseases, altered left ventricular (LV) geometry, and LV hypertrophy (LVH) [5,6,7].
Traditionally, conventional echocardiography is the most common imaging modality in detecting HHD by providing useful structural and hemodynamic findings. The presence of abnormal conventional echocardiographic findings, including abnormal LV geometry and LVH, dilatation of left atrium (LA), and LV systolic and diastolic dysfunction, is associated with poor prognosis associated with hypertension. Speckle-tracking echocardiography (STE) is a non-invasive echocardiographic modality providing myocardial deformation, which helps detect early ischemic changes and myocardial dysfunction, even before symptom onset, undetected by the conventional echocardiographic examination.
In this review article, we described the basic concept of STE and summarized the clinical utility of STE in the evaluation of patients with hypertension.
Structural and functional changes of the heart in hypertension
Because the target organ damage like HHD from arterial hypertension can be associated with a poor prognosis, identification of these HHD can improve clinical outcomes of these patients. In terms of biochemical alterations, myocardial fibrosis is one of the factors responsible for deterioration of myocardial function in hypertension [8, 9]. Myocardial fibrosis is a major determinant of LVH with the stiff myocardium in HHD, potentially leading to pump failure [10, 11]. Increased myocardial fibrosis and decreased elasticity are characterized by the accumulation of extracellular matrix proteins in the myocardium and surrounding microvasculatures [12]. Cardiac fibrosis alters the myocardial structures, resulting in hypertrophy, chamber stiffness, dilatation of cardiac chambers, conduction abnormalities and arrhythmogenicity, and systolic and diastolic dysfunction of the ventricles and aria [13].
LVH and abnormal LV geometry are common forms of HHD and important independent predictors of adverse long-term prognosis in many cardiovascular diseases. Chronic systolic and diastolic arterial hypertension can cause myocyte hypertrophy. And, hypertension can produce perivascular and myocardial fibrosis, and medial thickening of the intramyocardial coronary arteries [2]. Consequently, hypertension induces global modifications in the cardiac structure and function and is a well-recognized risk factor for developing HF and cardiovascular diseases [14,15,16]. Because the early detection of LV systolic dysfunction, even in patients without overt symptoms, can give opportunities to reverse their structural changes from hypertension, it is an important issue in hypertensive patients.
LA structural and functional changes can be observed in HHD [17]. Although LA remodeling is closely related to LV structural and functional alterations, it can occur independently from LV remodeling [17, 18]. Also, right ventricular (RV) remodeling can occur due to increased pulmonary arterial pressure. Arterial remodeling is also a common finding in HHD, and hypertension can cause increased stiffening of artery and aortic dilatation, which were associated with an increased risk of cardiovascular diseases [13, 19, 20].
Without prompt antihypertensive treatment, HF and arrhythmias can occur in patients with LVH and abnormal LV geometry. HF with reduced ejection fraction and HF with preserved ejection fraction can be observed in patients with HHD. Ischemic events, including myocardial ischemia and stroke, can also accompany.
What is myocardial strain and speckle-tracking echocardiography?
Myocardial fibers are a 3-dimensional structure including circumferential fibers in the mid-wall layer and longitudinal fibers in the endocardial and epicardial layers. Myofiber can stretch, shorten and thicken, and these movements in different myocardial layers change myofiber orientation continuously from right-handed helix in subendocardium to left-handed helix in subepicardium [21]. Thus, myocardial contraction occurs clockwise rotation in the midportion and counterclockwise rotation in the apical portion of the LV [22].
Left ventricular ejection fraction (LVEF) as conventional echocardiographic index does not represent intrinsic myocardial property. However, strain echocardiography, a newer echocardiographic modality, can measure myocardial deformation representing the intrinsic myocardial property.
Myocardial strain and strain rate
Myocardial strain is a dimensionless index that can represent myocardial performance. It can be calculated as the change from the original muscle length to the final muscle length after myocardial contraction and presented as a percentage (Fig. 2). During systole, myocardial fibers shorten, and strain value assessing fiber shortening has a negative value while lengthening of fibers during systole, often observed in the segment with dyskinesis, is represented by a positive strain value.
Myocardial strain and strain rate. Simple diagram showing the principle of strain and strain rate. Strain is expressed as a fractional length change, where shortening is a negative value and lengthening a positive value. Strain is calculated as the difference (ΔL) of the initial (L0) and the final distance (L) between two points divided by the initial distance. Strain rate is the deformation per unit time (Δt), and derives from the ratio between the velocity variation and the initial distance between two points
Strain rate considers the time to the strain and refers as a deformation per time unit. The unit of strain rate is s− 1 and has the same direction as the strain value, negative value during shortening and positive value during elongation of the myocardium (Fig. 2).
Speckle-tracking echocardiography
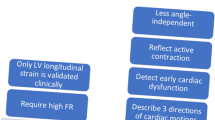
The myocardial strain and strain rate assessment is a good marker of myocardial function representing intrinsic myocardial performance [23,24,25,26,27]. Myocardial strain can be usually measured by 2-dimensional STE. It tracks ultrasonic speckles, small myocardial footprints in routine 2-dimensional echocardiographic images (Fig. 3). Myocardial speckles are tracked frame-by-frame in one cardiac cycle, and the strain was automatically calculated by measuring the distances between speckles [28]. Among myocardial deformation with three directions, including longitudinal strain (LS), circumferential strain (CS), and radial strain (RS), LS is the most frequently used deformation component from averaging values of 3 apical views in an easy way (Fig. 4).
Demonstration of a 2-dimensional strain analysis with GE EchoPAC PC software. After tracing of the endocardial border, the software provides global and regional myocardial strain values automatically in apical 4 chamber (A), apical 2 chamber (B), and apical 3 chamber views (C). The GE EchoPAC algorithm can provide bull’s eye maps of regional longitudinal strain values (D)
There are several factors that can affect left ventricular LS (LVLS) include hemodynamic factors, chamber geometry, myocardial tissue characteristics, and synchrony of myocardial contraction.
The increase in LV afterload or increasing heart rate can lead decrease in LVLS [29]. Also, the change of preload can be related to the change of LVLS [30]. Ventricular geometry can affect strain values. The increase of LV wall thickness and dilatation of LV cavity can be associated with the decrease in LS [31, 32]. Regional LS can be reduced in the areas of infiltrative or myocardial storage diseases [33]. Also, fibrotic myocardial areas, usually observed in the myocardial infarction, are associated with a decrease in regional LS. Inhomogeneous myocardial activation, usually associated with the left bundle branch block, is associated with the difference in sequence of myocardial contraction of the ventricular septum and the LV lateral wall. Other factors affecting LS include gender and age [34]. Usually, females have better LVLS values than males, and younger adults have better LS values than older adults.
STE can measure LA strain (Fig. 5A) and RV strain (Fig. 5B). Thus, STE has become a major echocardiographic modality measuring strain and strain rate in the clinical fields (Fig. 6).
Demonstration of LA and RV strain analysis using 2-dimensional speckle-tracking echocardiography (A). LA strain and illustration of the 3 phases of LA function with an R-R gating analysis. RV longitudinal strain calculated as the average of the six-segment model (B). ECG, electrocardiography; LA, left atrium; PACS, peak atrial contraction strain; PALS, peak atrial longitudinal strain; RV, right ventricular
Although echocardiographic strain assessment has relatively high inter-observer and intra-observer variability, as well as inter-vendor variability [35], it should be emphasized that tracking myocardial deformation in systole and diastole provides an additional quantitative measure of myocardial function. Several studies showed that LV global LS represented a better predictor of cardiovascular morbidity and mortality than LVEF in the general population, as well as in HF or various cardiovascular diseases [36,37,38,39]. LV multidirectional strain is also associated with LV diastolic function indices (E/e’ and E/A ratios), which means information about LV filling pressure [40, 41]. In addition, strain by STE has grown in importance in both the diagnostic and prognostic evaluation across the cardiac disease spectrum including coronary artery diseases, valvular heart diseases, cardiomyopathies, and RV dysfunction.
Clinical applications of speckle-tracking echocardiography in patients with hypertension
There are several clinical utilities of STE in patients with arterial hypertension (Fig. 6 and Table 1).
Hypertension patients with normal left ventricular geometry
It is well known that a preclinical LV systolic dysfunction occurs in hypertensive patients with LVH [44]. Even before LVH occurs, hypertension leads to early changes of LV mechanics. However, the conventional transthoracic echocardiography is usually unable to detect early subtle abnormalities in LV systolic function caused by arterial hypertension, prior to manifestation of LVH. The STE can identify subtle adaptive changes of LV systolic mechanics in hypertensive patients without symptoms or signs of HF and with normal contractile function. Patients with hypertension without LVH show impaired systolic LS compared with healthy control participants (− 18.0% ± 1.9% vs. –20.4% ± 2.5%, P = 0.02). In contrast, Doppler tissue imaging (DTI) was able to detect an LV systolic dysfunction only in the patients with LVH (P < 0.001) [42]. Moreover, in patients with LVH, STE showed lower LS (− 15.9% ± 3.3% vs. –20.4 ± 2.5%, P < 0.001) and RS (40% ± 20% vs. 54.5% ± 16%, P = 0.02), higher CS (− 22% ± 4% vs. –20% ± 2.8%, P = 0.05), and twist (23.8° ± 5.2° vs. 12.5° ± 4.8°, P < 0.001) than controls. Although basal LS by DTI was not evaluated, velocity measures were normal in hypertensive patients without LVH. This finding demonstrates the advantages of 2-dimensional strain over DTI in the early detection of LV systolic dysfunction. Early impairment of myocardial contractility may be secondary to hemodynamic or biochemical changes. The increased end-systolic wall stress plays a crucial role in leading to longitudinal dysfunction in HHD. Chronic increase in end-systolic wall stress promotes the subendocardial synthesis of collagen, reducing longitudinal deformation.
Kang et al. [43] revealed that impaired LS and increased LV torsion are associated with higher serum levels of tissue inhibitor of the metalloproteinase-1 matrix, which controls myocardial collagen turnover, in patients with hypertension and normal LVEF. They demonstrated that excessive accumulation of fibrillar collagen could progress to myocardial fibrosis and contribute to early LV contractile dysfunction. Because arterial hypertension has impact on all myocardial layers, endocardial, midcardial and epicardial LS were lower than controls, and epicardial LS was important predictor for cardiovascular events including cardiovascular mortality and rehospitalization in hypertensive population [45]. On the other hand, RS and CS, as well as twist, were preserved in hypertensive patients without structural LV changes in this study. These findings fit the novel criteria of HF classification, stating that only transmural damage was associated with reduction of LVEF, CS and twist, whereas subendocardial dysfunction results in isolated LS impairment [52].
Hypertensive patients with LVH
LVH is a common response to chronically elevated afterload, enabling normalization of LV wall stress and preservation of LV mechanical function [53]. LVH is an independent predictor of cardiovascular morbidity and mortality in hypertensive patients [54, 55]. This structural remodeling is associated with various alterations including myocardial stiffness, impaired vasomotor reactivity of coronary artery, depressed LV wall mechanics, and abnormal LV diastolic filling pattern [56,57,58,59,60].
A previous study indicated that longitudinal LV contractility was decreased in healthy elderly people, and in patients with hypertension, diabetes, hypertrophic cardiomyopathy, or diastolic HF [61,62,63,64]. The aging process promotes fibrosis of the subendocardial myocardium in normal subjects, and the connective tissue content increases in hypertensive patients with LVH [65, 66]. Myocardial fibrosis related to pressure overload is frequent in the subendocardial layer, and it is well known that there is a close association between abnormal longitudinal function and content of interstitial fibrosis [67]. Therefore, LV longitudinal function is deteriorated in healthy elderly people and in hypertension patients with or without LVH. In general, radial myocardial thickening is closely related to LV systolic function, and even if longitudinal LV function is deteriorated, radial function is still maintained. A previous study using 2-dimentional strain imaging reported that LV diastolic and systolic function were first impaired in the longitudinal direction in asymptomatic patients with cardiovascular risk factors and preserved LV pump function. In systole, radial thickening is preserved, and as the longitudinal shortening decreases, the circumferential shortening increases for maintenance of LVEF [47]. In addition, Mizuguchi et al. [44] indicated that concentric LVH caused systolic LV myocardial deformation in all 3 directions including longitudinal, circumferential, and radial in hypertensive patients. However, LV pump function were compensated by increasing circumferential shortening at ventricular systole. Thus, increase in circumferential shortening might be major compensatory mechanisms for maintaining LV pump function even when the longitudinal shortening is decreased.
Diastolic dysfunction in HHD
Age-related changes in LV relaxation have recently facilitated the detection of impaired LV diastolic function as reflected by E/A < 1 and a prolonged deceleration time of early diastolic transmitral flow with the widespread use of pulsed Doppler echocardiography. Several studies reported that diastolic LV dysfunction precedes systolic LV dysfunction especially in patients with cardiovascular risk factors such as hyperlipidemia, diabetes, hypertension, obesity, and smoking habits [68,69,70,71,72]. LV multidirectional strain is associated with LV diastolic function indices such as E/e’ and E/A ratios that enables information regarding LV filling pressure [40, 41, 73]. In addition, strain rates (early and late diastolic and systolic) represent strain equivalent for tissue Doppler-derived parameters and correspond well with indices of LV diastolic function. In diastole, the mean peak early diastolic longitudinal strain rate decreased in longitudinal and radial directions, particularly in the former direction. There was a positive correlation between the longitudinal early diastolic strain rate and E/A. In addition, the peak LV longitudinal strain rate during atrial systole was an independent predictor related to E/A in all patients [46, 47]. However, there was no significant increase in pressure at the end of the LV diastole because sufficient LV filling was achieved by effective atrial contraction. Therefore, longitudinal LV myocardial deformation is an important marker to detect subclinical changes in LV contraction and relaxation. Because LA structure and function are directly influenced by the LV filling pressure, LA assessment is an essential step in the diagnosis of diastolic dysfunction. LA strain derived from STE can help assess LA function objectively through the 3 distinct phasic motions of the LA cycle, which are significantly influenced by the LV diastolic strain rates and LV global LS.
Previous study demonstrated that hypertension is directly associated with LA volume change, and it has been demonstrated that despite normal LA volume, there is abnormal LALS in hypertension [74]. This shows that the earliest abnormality in hypertension appear as a decrease in LA strain, followed by LA dilatation, subclinical LV dysfunction, and finally HF with normal or reduced LVEF [2]. Salas Pacheco et al. [48] found that in patients with HHD, the indexed LA volume was greater than in the control group (34 ± 7.8 mL/m2 vs. 24 ± 4.9 mL/m2); strain of pump (− 5.7% ± 2.4% vs. − 17% ± 3.5%) and reservoir phases (34% ± 9% vs. 48% ± 10%) were worst. The minimum LA volume was higher (26 ± 10 mL vs. 15 ± 8 mL) and LA independent strain was lower in hypertensive patients (4.0% vs. 6.5%, P = 0.001). The LA independent strain quantifies LA reservoir phase deformation during isovolumetric relaxation. These findings suggest that in the early stages of HHD, the LA experiences a remodeling with dilation and reduction of LS in the pump and reservoir phase while sustains normal LVEF and global LS. LA strain represents a new tool in the evaluation of atrial function. STE is more sensitive method to initial changes in myocardial function and independent of the insonation angle, but since the absolute value is affected by the hemodynamic loading condition, the evaluation of LA strain during isovolumic relaxation may reflects a condition independent of the hemodynamic loading and traction by the atrioventricular plain [75]. In addition, the lower value of independent strain in hypertensive patients shows that there is intrinsic atrial myocyte damage with significant dysfunction in the early stages [48].
RV function in HHD
Arterial hypertension can impact pulmonary circulation and change pulmonary arterial structure. Sequentially, these can provoke RV remodeling. Common pathophysiologic mechanisms include common pathways of arterial hypertension and impaired LV diastolic function as results of increased LV afterload. Overstimulation of the sympathetic system and the renin—angiotensin—aldosterone system can alter pulmonary vascular structure and increase pulmonary vascular resistance. Increased pulmonary vascular resistance impairs RV function and causes RV hypertrophy, subsequently [76]. Arterial hypertension induces LV hypertrophy and cause LV diastolic dysfunction. In patients with LV diastolic dysfunction, increased LA pressure can be delivered to pulmonary capillary and pulmonary artery. Prolonged increased LA pressure can induce pulmonary hypertension as results. Increased RV filling pressure, RV hypertrophy and ventricular interdependency can cause RV systolic and diastolic dysfunction in patients with arterial hypertension.
Conventional echocardiographic study is insufficient in the demonstration of RV dysfunction in patients with arterial hypertension in several studies [50, 77,78,79]. However, strain echocardiography can show subtle changes of RV. In the early stage of RV remodeling, RV longitudinal function is progressively decreased, but transversal function is preserved and even increased due to circumferential fibers of the subepicardial layer. Therefore, compared to the fractional area change remaining within the normal range for a long time and deteriorates last in the cascade, RV LS can be a sensitive parameter of RV function capable of detecting subtle changes at subclinical levels in patients with arterial hypertension. In the first study using speckle-tracking imaging in young hypertensive patients, RV strain and strain rates were deteriorated particularly in apical and mid segments of RV free wall than in the basal RV segment [49]. Tumuklu et al. [50] reported that free wall RV peak systolic strain was significantly lower in both groups of hypertensive patients with and without LVH than in the normotensive controls. The study results of patients with prehypertension revealed that RV global LS was gradually decreased from the subjects with optimal blood pressure, across the prehypertensive subjects to the hypertensive individuals [80]. Tadic et al. [51] showed that untreated hypertensive patients have significantly lower RV global LS compared to well-regulated hypertensive patients and controls, which was similar to poorly controlled hypertensive patients. Furthermore, functional capacity estimated by peak oxygen consumption correlated with RV free wall LS, 3-dimensional RV end-diastolic volume, RV stroke volume, RV ejection fraction, and right atrial LS. However, only RV free wall strain and 3-dimensional RV stroke volume were independently associated with peak oxygen uptake in the study population.
Conclusion
Hypertension is a well-recognized risk factor for the development of cardiovascular disease. Preclinical LV systolic dysfunction occurs in hypertensive patients and LVH, but even before LVH occurs, hypertension causes early changes in LV dynamics. Therefore, the accurate assessment of their influence on LV systolic and diastolic function in a subclinical state is clinically important for preventing the development of overt cardiovascular diseases. STE can detect subtle changes in myocardial dysfunction that cannot be detected with conventional echocardiography before the overt manifestation of LVH appear.
Both longitudinal LV diastolic and systolic function can be impaired early even in asymptomatic patients with cardiovascular risk factors and preserved LV systolic function. In systolic phase, longitudinal shortening decreases and radial thickening was preserved, but the circumferential shortening increases to maintain the LVEF. In the diastolic phase, the early diastolic strain rate decreased, especially in the longitudinal direction, even if the LV filling pressure did not increase significantly. In this regard, strain appears to be more sensitive than both conventional echocardiography and DTI in identifying a decrease of intrinsic myocardial contractility in hypertensive patients even long before LVH occurs. Thus, the assessment of myocardial contractility by the STE will be useful in the early detection of subclinical systolic dysfunction and change of LV mechanics. Echocardiographic strain provides more insight into subtle changes in LV function in terms of identifying patients at higher risk for HF and clarifying its clinical impact on the prognosis of HHD.
Availability of data and materials
Not applicable.
Abbreviations
- CS:
-
Circumferential strain
- DTI:
-
Doppler tissue imaging
- HF:
-
Heart failure
- HHD:
-
Hypertensive heart diseases
- LA:
-
Left atrium
- LS:
-
Longitudinal strain
- LV:
-
Left ventricular
- LVEF:
-
Left ventricular ejection fraction
- LVH:
-
Left ventricular hypertrophy
- LVLS:
-
Left ventricular longitudinal strain
- RS:
-
Radial strain
- RV:
-
Right ventricular
- STE:
-
Speckle-tracking echocardiography
References
Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, et al. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation. 2020;141(9):e139–596. https://doi.org/10.1161/CIR.0000000000000757.
Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123(3):327–34. https://doi.org/10.1161/CIRCULATIONAHA.108.845792.
Messerli FH, Williams B, Ritz E. Essential hypertension. Lancet. 2007;370(9587):591–603. https://doi.org/10.1016/S0140-6736(07)61299-9.
Tocci G, Sciarretta S, Volpe M. Development of heart failure in recent hypertension trials. J Hypertens. 2008;26(7):1477–86. https://doi.org/10.1097/HJH.0b013e3282fe1d3d.
Levy D, Garrison RJ, Savage DD, Kannel WB, Castelli WP. Prognostic implications of echocardiographically determined left ventricular mass in the Framingham heart study. N Engl J Med. 1990;322(22):1561–6. https://doi.org/10.1056/NEJM199005313222203.
Koren MJ, Devereux RB, Casale PN, Savage DD, Laragh JH. Relation of left ventricular mass and geometry to morbidity and mortality in uncomplicated essential hypertension. Ann Intern Med. 1991;114(5):345–52. https://doi.org/10.7326/0003-4819-114-5-345.
Verdecchia P, Schillaci G, Borgioni C, Ciucci A, Battistelli M, Bartoccini C, et al. Adverse prognostic significance of concentric remodeling of the left ventricle in hypertensive patients with normal left ventricular mass. J Am Coll Cardiol. 1995;25(4):871–8. https://doi.org/10.1016/0735-1097(94)00424-O.
Komuro I, Katoh Y, Kaida T, Shibazaki Y, Kurabayashi M, Hoh E, et al. Mechanical loading stimulates cell hypertrophy and specific gene expression in cultured rat cardiac myocytes. Possible role of protein kinase C activation. J Biol Chem. 1991;266(2):1265–8. https://doi.org/10.1016/S0021-9258(17)35310-3.
Brilla CG, Janicki JS, Weber KT. Impaired diastolic function and coronary reserve in genetic hypertension. Role of interstitial fibrosis and medial thickening of intramyocardial coronary arteries. Circ Res. 1991;69(1):107–15. https://doi.org/10.1161/01.RES.69.1.107.
Weber KT, Brilla CG, Janicki JS. Myocardial fibrosis: functional significance and regulatory factors. Cardiovasc Res. 1993;27(3):341–8. https://doi.org/10.1093/cvr/27.3.341.
Alla F, Kearney-Schwartz A, Radauceanu A, Das Dores S, Dousset B, Zannad F. Early changes in serum markers of cardiac extra-cellular matrix turnover in patients with uncomplicated hypertension and type II diabetes. Eur J Heart Fail. 2006;8(2):147–53. https://doi.org/10.1016/j.ejheart.2005.06.008.
Humeres C, Frangogiannis NG. Fibroblasts in the infarcted, remodeling, and failing heart. JACC Basic Transl Sci. 2019;4(3):449–67. https://doi.org/10.1016/j.jacbts.2019.02.006.
Nwabuo CC, Vasan RS. Pathophysiology of hypertensive heart disease: beyond left ventricular hypertrophy. Curr Hypertens Rep. 2020;22(2):11. https://doi.org/10.1007/s11906-020-1017-9.
Haider AW, Larson MG, Franklin SS, Levy D, Framingham Heart Study. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138:10–6.
Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996;275(20):1557–62. https://doi.org/10.1001/jama.1996.03530440037034.
Kannel WB, Castelli WP, McNamara PM, McKee PA, Feinleib M. Role of blood pressure in the development of congestive heart failure. The Framingham study. N Engl J Med. 1972;287(16):781–7. https://doi.org/10.1056/NEJM197210192871601.
Thomas L, Abhayaratna WP. Left atrial reverse remodeling: mechanisms, evaluation, and clinical significance. JACC Cardiovasc Imaging. 2017;10(1):65–77. https://doi.org/10.1016/j.jcmg.2016.11.003.
McManus DD, Xanthakis V, Sullivan LM, Zachariah J, Aragam J, Larson MG, et al. Longitudinal tracking of left atrial diameter over the adult life course: clinical correlates in the community. Circulation. 2010;121(5):667–74. https://doi.org/10.1161/CIRCULATIONAHA.109.885806.
Lam CS, Gona P, Larson MG, Aragam J, Lee DS, Mitchell GF, et al. Aortic root remodeling and risk of heart failure in the Framingham heart study. JACC Heart Fail. 2013;1(1):79–83. https://doi.org/10.1016/j.jchf.2012.10.003.
Tsao CW, Lyass A, Larson MG, Levy D, Hamburg NM, Vita JA, et al. Relation of central arterial stiffness to incident heart failure in the community. J Am Heart Assoc. 2015;4:e002189.
Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1(3):366–76. https://doi.org/10.1016/j.jcmg.2008.02.006.
Buckberg G, Hoffman JI, Mahajan A, Saleh S, Coghlan C. Cardiac mechanics revisited: the relationship of cardiac architecture to ventricular function. Circulation. 2008;118(24):2571–87. https://doi.org/10.1161/CIRCULATIONAHA.107.754424.
Cikes M, Solomon SD. Beyond ejection fraction: an integrative approach for assessment of cardiac structure and function in heart failure. Eur Heart J. 2016;37(21):1642–50. https://doi.org/10.1093/eurheartj/ehv510.
Haugaa KH, Dejgaard LA. Global longitudinal strain: ready for clinical use and guideline implementation. J Am Coll Cardiol. 2018;71(18):1958–9. https://doi.org/10.1016/j.jacc.2018.03.015.
Park JJ, Park JB, Park JH, Cho GY. Global longitudinal strain to predict mortality in patients with acute heart failure. J Am Coll Cardiol. 2018;71(18):1947–57. https://doi.org/10.1016/j.jacc.2018.02.064.
Urheim S, Edvardsen T, Torp H, Angelsen B, Smiseth OA. Myocardial strain by Doppler echocardiography. Validation of a new method to quantify regional myocardial function. Circulation. 2000;102(10):1158–64. https://doi.org/10.1161/01.CIR.102.10.1158.
Edvardsen T, Gerber BL, Garot J, Bluemke DA, Lima JA, Smiseth OA. Quantitative assessment of intrinsic regional myocardial deformation by Doppler strain rate echocardiography in humans: validation against three-dimensional tagged magnetic resonance imaging. Circulation. 2002;106(1):50–6. https://doi.org/10.1161/01.CIR.0000019907.77526.75.
Collier P, Phelan D, Klein A. A test in context: myocardial strain measured by speckle-tracking echocardiography. J Am Coll Cardiol. 2017;69(8):1043–56. https://doi.org/10.1016/j.jacc.2016.12.012.
Donal E, Bergerot C, Thibault H, Ernande L, Loufoua J, Augeul L, et al. Influence of afterload on left ventricular radial and longitudinal systolic functions: a two-dimensional strain imaging study. Eur J Echocardiogr. 2009;10(8):914–21. https://doi.org/10.1093/ejechocard/jep095.
Negishi K, Borowski AG, Popovic ZB, Greenberg NL, Martin DS, Bungo MW, et al. Effect of gravitational gradients on cardiac filling and performance. J Am Soc Echocardiogr. 2017;30(12):1180–8. https://doi.org/10.1016/j.echo.2017.08.005.
Reant P, Metras A, Detaille D, Reynaud A, Diolez P, Jaspard-Vinassa B, et al. Impact of afterload increase on left ventricular myocardial deformation indices. J Am Soc Echocardiogr. 2016;29(12):1217–8. https://doi.org/10.1016/j.echo.2016.09.006.
Marciniak A, Claus P, Sutherland GR, Marciniak M, Karu T, Baltabaeva A, et al. Changes in systolic left ventricular function in isolated mitral regurgitation. A strain rate imaging study. Eur Heart J. 2007;28(21):2627–36. https://doi.org/10.1093/eurheartj/ehm072.
Voigt JU, Cvijic M. 2- and 3-dimensional myocardial strain in cardiac health and disease. JACC Cardiovasc Imaging. 2019;12(9):1849–63. https://doi.org/10.1016/j.jcmg.2019.01.044.
Park JH, Lee JH, Lee SY, Choi JO, Shin MS, Kim MJ, et al. Normal 2-dimensional strain values of the left ventricle: a substudy of the normal echocardiographic measurements in Korean population study. J Cardiovasc Ultrasound. 2016;24(4):285–93. https://doi.org/10.4250/jcu.2016.24.4.285.
Farsalinos KE, Daraban AM, Ünlü S, Thomas JD, Badano LP, Voigt JU. Head-to-head comparison of global longitudinal strain measurements among nine different vendors: the EACVI/ASE inter-vendor comparison study. J Am Soc Echocardiogr. 2015;28(10):1171–81. https://doi.org/10.1016/j.echo.2015.06.011.
Biering-Sørensen T, Biering-Sørensen SR, Olsen FJ, Sengeløv M, Jørgensen PG, Mogelvang R, et al. Global longitudinal strain by echocardiography predicts long-term risk of cardiovascular morbidity and mortality in a low-risk general population: the Copenhagen city heart study. Circ Cardiovasc Imaging. 2017;10(3):e005521. https://doi.org/10.1161/CIRCIMAGING.116.005521.
Sengeløv M, Jørgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8(12):1351–9. https://doi.org/10.1016/j.jcmg.2015.07.013.
Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132(5):402–14. https://doi.org/10.1161/CIRCULATIONAHA.115.015884.
Saito M, Khan F, Stoklosa T, Iannaccone A, Negishi K, Marwick TH. Prognostic implications of LV strain risk score in asymptomatic patients with hypertensive heart disease. JACC Cardiovasc Imaging. 2016;9(8):911–21. https://doi.org/10.1016/j.jcmg.2015.09.027.
Soufi Taleb Bendiab N, Meziane-Tani A, Ouabdesselam S, Methia N, Latreche S, Henaoui L, et al. Factors associated with global longitudinal strain decline in hypertensive patients with normal left ventricular ejection fraction. Eur J Prev Cardiol. 2017;24(14):1463–72. https://doi.org/10.1177/2047487317721644.
Mochizuki Y, Tanaka H, Matsumoto K, Sano H, Shimoura H, Ooka J, et al. Impact of left ventricular longitudinal functional mechanics on the progression of diastolic function in diabetes mellitus. Int J Cardiovasc Imaging. 2017;33(12):1905–14. https://doi.org/10.1007/s10554-017-1198-8.
Imbalzano E, Zito C, Carerj S, Oreto G, Mandraffino G, Cusmà-Piccione M, et al. Left ventricular function in hypertension: new insight by speckle tracking echocardiography. Echocardiography. 2011;28(6):649–57. https://doi.org/10.1111/j.1540-8175.2011.01410.x.
Kang SJ, Lim HS, Choi BJ, Choi SY, Hwang GS, Yoon MH, et al. Longitudinal strain and torsion assessed by two-dimensional speckle tracking correlate with the serum level of tissue inhibitor of matrix metalloproteinase-1, a marker of myocardial fibrosis, in patients with hypertension. J Am Soc Echocardiogr. 2008;21(8):907–11. https://doi.org/10.1016/j.echo.2008.01.015.
Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. Concentric left ventricular hypertrophy brings deterioration of systolic longitudinal, circumferential, and radial myocardial deformation in hypertensive patients with preserved left ventricular pump function. J Cardiol. 2010;55(1):23–33. https://doi.org/10.1016/j.jjcc.2009.07.006.
Lee WH, Liu YW, Yang LT, Tsai WC. Prognostic value of longitudinal strain of subepicardial myocardium in patients with hypertension. J Hypertens. 2016;34(6):1195–200. https://doi.org/10.1097/HJH.0000000000000903.
Mu Y, Qin C, Wang C, Huojiaabudula G. Two-dimensional ultrasound speckle tracking imaging in evaluation of early changes in left ventricular diastolic function in patients with essential hypertension. Echocardiography. 2010;27(2):146–54. https://doi.org/10.1111/j.1540-8175.2009.00984.x.
Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr. 2008;21(10):1138–44. https://doi.org/10.1016/j.echo.2008.07.016.
Salas Pacheco JL, Sánchez OL. Independent parameters of left atrium function in hypertensive heart disease. Echocardiography. 2019;36(12):2195–201. https://doi.org/10.1111/echo.14542.
Pedrinelli R, Canale ML, Giannini C, Talini E, Dell'Omo G, Di Bello V. Abnormal right ventricular mechanics in early systemic hypertension: a two-dimensional strain imaging study. Eur J Echocardiogr. 2010;11(9):738–42. https://doi.org/10.1093/ejechocard/jeq059.
Tumuklu MM, Erkorkmaz U, Ocal A. The impact of hypertension and hypertension-related left ventricle hypertrophy on right ventricle function. Echocardiography. 2007;24(4):374–84. https://doi.org/10.1111/j.1540-8175.2007.00419.x.
Tadic M, Cuspidi C, Suzic-Lazic J, Andric A, Stojcevski B, Ivanovic B, et al. Is there a relationship between right-ventricular and right atrial mechanics and functional capacity in hypertensive patients. J Hypertens. 2014;32(4):929–37. https://doi.org/10.1097/HJH.0000000000000102.
Sengupta PP, Narula J. Reclassifying heart failure: predominantly subendocardial, subepicardial, and transmural. Heart Fail Clin. 2008;4(3):379–82. https://doi.org/10.1016/j.hfc.2008.03.013.
Frohlich ED, Apstein C, Chobanian AV, Devereux RB, Dustan HP, Dzau V, et al. The heart in hypertension. N Engl J Med. 1992;327(14):998–1008. https://doi.org/10.1056/NEJM199210013271406.
Casale PN, Devereux RB, Milner M, Zullo G, Harshfield GA, Pickering TG, et al. Value of echocardiographic measurement of left ventricular mass in predicting cardiovascular morbid events in hypertensive men. Ann Intern Med. 1986;105(2):173–8. https://doi.org/10.7326/0003-4819-105-2-173.
Devereux RB, Wachtell K, Gerdts E, Boman K, Nieminen MS, Papademetriou V, et al. Prognostic significance of left ventricular mass change during treatment of hypertension. JAM A. 2004;292(19):2350–6. https://doi.org/10.1001/jama.292.19.2350.
Doering CW, Jalil JE, Janicki JS, Pick R, Aghili S, Abrahams C, et al. Collagen network remodelling and diastolic stiffness of the rat left ventricle with pressure overload hypertrophy. Cardiovasc Res. 1988;22(10):686–95. https://doi.org/10.1093/cvr/22.10.686.
Jalil JE, Doering CW, Janicki JS, Pick R, Clark WA, Abrahams C, et al. Structural vs. contractile protein remodeling and myocardial stiffness in hypertrophied rat left ventricle. J Mol Cell Cardiol. 1988;20(12):1179–87. https://doi.org/10.1016/0022-2828(88)90597-4.
Wicker P, Tarazi RC, Kobayashi K. Coronary blood flow during the development and regression of left ventricular hypertrophy in renovascular hypertensive rats. Am J Cardiol. 1983;51(10):1744–9. https://doi.org/10.1016/0002-9149(83)90222-9.
Canby CA, Tomanek RJ. Role of lowering arterial pressure on maximal coronary flow with and without regression of cardiac hypertrophy. Am J Phys. 1989;257(4):H1110–8. https://doi.org/10.1152/ajpheart.1989.257.4.H1110.
Haider AW, Larson MG, Benjamin EJ, Levy D. Increased left ventricular mass and hypertrophy are associated with increased risk for sudden death. J Am Coll Cardiol. 1998;32(5):1454–9. https://doi.org/10.1016/S0735-1097(98)00407-0.
Mishiro Y, Oki T, Yamada H, Onose Y, Matsuoka M, Tabata T, et al. Use of angiotensin II stress pulsed tissue Doppler imaging to evaluate regional left ventricular contractility in patients with hypertrophic cardiomyopathy. J Am Soc Echocardiogr. 2000;13(12):1065–73. https://doi.org/10.1067/mje.2000.111010.
Koulouris SN, Kostopoulos KG, Triantafyllou KA, Karabinos I, Bouki TP, Karvounis HI, et al. Impaired systolic dysfunction of left ventricular longitudinal fibers: a sign of early hypertensive cardiomyopathy. Clin Cardiol. 2005;28(6):282–6. https://doi.org/10.1002/clc.4960280605.
Fang ZY, Leano R, Marwick TH. Relationship between longitudinal and radial contractility in subclinical diabetic heart disease. Clin Sci (Lond). 2004;106(1):53–60. https://doi.org/10.1042/CS20030153.
Yu CM, Lin H, Yang H, Kong SL, Zhang Q, Lee SW. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105(10):1195–201. https://doi.org/10.1161/hc1002.105185.
Lenkiewicz JE, Davies MJ, Rosen D. Collagen in human myocardium as a function of age. Cardiovasc Res. 1972;6(5):549–55. https://doi.org/10.1093/cvr/6.5.549.
Pearlman ES, Weber KT, Janicki JS, Pietra GG, Fishman AP. Muscle fiber orientation and connective tissue content in the hypertrophied human heart. Lab Investig. 1982;46(2):158–64.
Lumens J, Delhaas T, Arts T, Cowan BR, Young AA. Impaired subendocardial contractile myofiber function in asymptomatic aged humans, as detected using MRI. Am J Physiol Heart Circ Physiol. 2006;291(4):H1573–9. https://doi.org/10.1152/ajpheart.00074.2006.
de las Fuentes L, Waggoner AD, Brown AL, Dávila-Román VG. Plasma triglyceride level is an independent predictor of altered left ventricular relaxation. J Am Soc Echocardiogr. 2005;18(12):1285–91. https://doi.org/10.1016/j.echo.2005.05.002.
Ahmed SS, Jaferi GA, Narang RM, Regan TJ. Preclinical abnormality of left ventricular function in diabetes mellitus. Am Heart J. 1975;89(2):153–8. https://doi.org/10.1016/0002-8703(75)90039-3.
Fouad FM, Slominski JM, Tarazi RC. Left ventricular diastolic function in hypertension: relation to left ventricular mass and systolic function. J Am Coll Cardiol. 1984;3(6):1500–6. https://doi.org/10.1016/S0735-1097(84)80289-2.
Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B, et al. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43(8):1399–404. https://doi.org/10.1016/j.jacc.2003.10.062.
Alam M, Samad BA, Wardell J, Andersson E, Höglund C, Nordlander R. Acute effects of smoking on diastolic function in healthy participants: studies by conventional doppler echocardiography and doppler tissue imaging. J Am Soc Echocardiogr. 2002;15(10):1232–7. https://doi.org/10.1067/mje.2002.124006.
Hayashi T, Yamada S, Iwano H, Nakabachi M, Sakakibara M, Okada K, et al. Left ventricular global strain for estimating relaxation and filling pressure - a multicenter study. Circ J. 2016;80(5):1163–70. https://doi.org/10.1253/circj.CJ-16-0106.
Vaziri SM, Larson MG, Lauer MS, Benjamin EJ, Levy D. Influence of blood pressure on left atrial size: The Framingham Heart Study. Hypertension. 1995;25(6):1155–60. https://doi.org/10.1161/01.HYP.25.6.1155.
Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr. 2011;24(3):277–313. https://doi.org/10.1016/j.echo.2011.01.015.
Schermuly RT, Ghofrani HA, Wilkins MR, Grimminger F. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol. 2011;8(8):443–55. https://doi.org/10.1038/nrcardio.2011.87.
Pedrinelli R, Canale ML, Giannini C, Talini E, Penno G, Dell’Omo G, et al. Right ventricular dysfunction in early systemic hypertension: a tissue Doppler imaging study in patients with high-normal and mildly increased arterial blood pressure. J Hypertens. 2010;28(3):615–21. https://doi.org/10.1097/HJH.0b013e328334f181.
Cicala S, Galderisi M, Caso P, Petrocelli A, D’Errico A, de Divitiis O, et al. Right ventricular diastolic dysfunction in arterial systemic hypertension: analysis by pulsed tissue Doppler. Eur J Echocardiogr. 2002;3(2):135–42. https://doi.org/10.1053/euje.2001.0124.
Karaye KM, Sai’du H, Shehu MN. Right ventricular dysfunction in a hypertensive population stratified by patterns of left ventricular geometry. Cardiovasc J Afr. 2012;23(9):478–82. https://doi.org/10.5830/CVJA-2012-014.
Tadic M, Cuspidi C, Pencic B, Sljivic A, Ivanovic B, Neskovic A, et al. High-normal blood pressure impacts the right heart mechanics: a three-dimensional echocardiography and two-dimensional speckle tracking imaging study. Blood Press Monit. 2014;19(3):145–52. https://doi.org/10.1097/MBP.0000000000000043.
Acknowledgements
We thank to Sung-Won Park for her excellent illustration.
Funding
There was no funding source.
Author information
Authors and Affiliations
Contributions
All authors participated in the conception of the study. OJK and PJH: study conception and design, acquisition of data, interpretation of data, drafting of manuscript, and critical revision. Both authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
Jin Kyung Oh has nothing to declare. Jae-Hyeong Park serves on the editorial boards as a deputy editor in the Clinical Hypertension.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Oh, J.K., Park, JH. Role of strain echocardiography in patients with hypertension. Clin Hypertens 28, 6 (2022). https://doi.org/10.1186/s40885-021-00186-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40885-021-00186-y