Abstract
Since European settlement more than 10 % of Australia’s native fauna have become extinct and the current picture reflects 46 % are at various vulnerability stages. Australia’s iconic marsupial species, koala (Phascolarctos cinereus) is listed as vulnerable under national environmental law. Human population growth, road expansion and extensive land clearance have fragmented their eucalyptus habitat and reduced the ability of koalas to move across the tree canopy; making the species most vulnerable on the ground. Disease-principally chlamydia, road death, dog-attack and loss of habitat are key environmental pressures and the reasons why koalas are admitted for veterinary care. It is important to understand the dynamics of the physiological impacts that the koala faces from anthropogenic induced environmental challenges, especially on its essential biological functions (e.g. reproduction and immune system function). This review explores published literature and clinical data to identify key environmental stressors that are operating in mainland koala habitats, and while the focus is mostly on the koala, much of the information is analogous to other wildlife; the review may provide the impetus for future investigations involving other vulnerable native wildlife species (e.g. frogs). Oxalate nephrosis associated renal failure appears to be the most prevalent disease in koala populations from South Australia. Other key environmental stressors included heat stress, car impacts and dog attacks. It is possible that maternal stress, nutritional deprivation, dehydration and possible accumulation of oxalate in eucalyptus leaf increase mostly during drought periods impacting on fetal development. We hypothesize that chronic stress, particularly in urban and fringe zones, is creating very large barriers for conservation and recovery programs. Chronic stress in koalas is a result of the synergistic interplay between proximate environmental stressor/s (e.g. heat stress and fringe effects) acting on the already compromised kidney function, immune- and reproductive suppression. Furthermore, the effects of environmental pollutants in the aggravation of diseases such as kidney failure, reproductive suppression and suppression of the unique marsupial immune system should be researched. Environmental policies should be strengthened to increase human awareness of the threats facing the koala, increased funding support towards scientific research and the protection and creation of reserve habitats in urban areas and fringe zones. Global climate change, nutritional deprivation (loss of food sources), inappropriate fire management, invasive species and the loss of genetic diversity represent the complexities of environmental challenges impacting the koala biology.
Similar content being viewed by others
Background
Human population growth and global climate change are severely impacting on Australia’s environment [1–4]. The negative effects of climate change are clearly demonstrated in the steady decline of native Australian wildlife [5, 6]. Australia is home to a distinctive and robust range of many endemic fauna [4], including the koala (Phascolarctos cinereus). To safeguard the future of native wildlife species, it is crucial for researchers to identify the biological impacts that environmental stressors have on animals, hindering their ability to reproduce and survive. In this review, we will use the example of the koala to provide a detailed discussion on the biological impacts of key environmental stressors on wildlife in Australia.
Stress or any disturbance in an animal’s natural surrounding activates a ‘flight or fight’ response; a complex cascade of neurohormonal events [7, 8], including an acute stress response (releasing glucocorticoids-a group of corticosteroids with metabolic actions). Glucocorticoids direct energy towards a physical effort [8, 9] and it is an adaptive survival response [9, 10]. On the contrary, chronic stress (prolonged exposure to any noxious stimulus; predation for example) leads to sensitized glucocorticoid reactivity to stressors (See excellent review by [11]), which could have an inhibitory effect on reproductive hormone secretion ([8, 12]) affecting reproductive organ functionality [7], testicular histology [12, 13] and reproductive success [14]. Furthermore, a compromised immune system [7, 15] concomitantly may expose the animal to increased risk of autoimmune diseases, viral infections/resurgence [16], endotoxic shock [17] and susceptibility to parasitism/wound infections [18].
Mortality events in the koala are reflected similarly in other Australian wildlife [19–22] and include predation by feral/introduced species [4, 23], loss of habitat/fragmentation/suitable trees [24–30], climate change [4], car impacts [31], inappropriate fire management [20], drought/extreme temperature events [4, 32], pollutants [33, 34] and disease [30, 35–39]. Chlamydia is a major contributor within the decline of the koala [38, 40] and together with the Koala retrovirus (KoRV) these are the two major endemic disease manifestations in wild and captive koalas [39, 41]. As in other species, manifestations of disease incidence are strongly associated with unpredictable environmental change [27, 28, 36, 41, 42] reflecting a common environmental stress related impact.
The primary objective of this review paper is to scrutinize both clinical data and published literature to identify key environmental stressors and their biological impacts on koala populations.
Environmental stressors
The negative effects of anthropomorphic driven environmental change (e.g. extreme heat wave events) on wildlife species are increasing [5]. There are consequences of these changes to the physical and biological systems due to environmental stressors, such as wildlife road deaths [2] or pathogen spill over [43–45]. The complexity of the environmental interactions leading to these occurrences can be synergistic and multi-directional. Urbanisation and increasing population spread often have abrupt and tangible effects on the environment; divergence of water sources [46], clearance of habitats for agriculture [47] and logging of forests [35, 48]. Less tangible are the malignant effects of climate change [49–51] and environmental contamination from pollution [33, 52, 53]. Greater aridity, erosion and extreme weather events driven by anthropogenic climate change [54–57] decrease environmental productivity [47] and increase competition between native and exotic herbivores [6, 58]. Competition between introduced and native carnivores [59] significantly alter the predator–prey dyad [60] allowing exotic carnivorous species to gain pre-eminence and prey on native species with increased rapacity [60–63]. Food availability decreases not only for native herbivores but also for any species that relies on a degraded biome [64]. Interspecies competition for nutrition [59, 64, 65] and suitable breeding niches [64] often preface failure of immune functions [15, 48] and reproductive hormone suppression [66] limiting, at every level, species’ ability to fulfil their biological imperative. Fungicides, pesticides [52], perfluorinated compounds and radionuclides [33, 53] persist in the environment [52] and bio-accumulate in the food chain [33, 53, 67]. Reprotoxic pollutants have a multi-faceted interaction with habitat degradation [52] and as a limiting factor within species’ reproductive success [68–73]. Wildlife road death appears to be a simple case of cause and effect, but in reality may be the ultimate representation of multiple, synergistic, complex dynamic environmental processes. Hypothetically, the death of a koala may represent a search for food [65], or it could be related to cognitive or psychological malfunction from disease [74] or parasitism ([75–77], [76, 78–83]), be searching for a mate or suitable breeding opportunity [64] or simply trying to escape from an introduced predator species [84–86] or controlled burn off [87]. The selection pressures affecting Australian wildlife are unprecedented [3]. When faced with these challenges species may respond by relocating to a suitable biome. This is clearly demonstrated in the pattern of movement of many native passerines tracking a suitable climatic niche, before major habitat destruction, across arid areas of Australia [88–91]. A species may alternatively show phenotypic plasticity [3] in response to environmental changes and remain within their traditional biome [92] or undergo genetic change in evolutionary time-scale. Evidence of adaptive genetic change is few although Gardner et al. [93] presented strong evidence for genetically driven morphological changes. Environmental degradation and fragmentation within a rapid spatial and temporal pattern limits the species’ adaptive ability [48] at the velocity required for species longevity [3]. Isolation of populations as a result of habitat degradation, predation [94] or populations devastated during extreme weather events [95] can impact on species genetic diversity limiting their potential for evolution [94] or recovery when a stressor has subsided.
Evidence suggests that the velocity of climate change within this century is expected to be rapid [96] with a collateral need of species to adapt and redistribute at a pace commensurate with the movement of suitable climates. To calculate the velocity of climate change, global temperature has been used [96] with evidence that a wide species range will/have shift/ed poleward or uphill in response to increased global temperatures [1, 49, 97, 98]. From the late 19th century global temperatures have risen by 0.6 °C with an acceleration of increase recorded in the last 40 years [93] however, these studies have focused on temperate geographical climates [49, 98]. Walther et al. [51] commented that biotic and abiotic ecosystems are not governed by world-wide temperature averages and sub-regional variation is of greater relevance in assessment of the ecological impacts of climate change. Van Der Wal et al. [91]) analysed 60 years of climatic data showing that the dispersal of Australian wildlife often orientated towards increased temperature in response to precipitation events. The findings of Van Der Wal et al. [91] when viewed in the context of the unique Australian weather patterns seem hardly surprising. Australia historically has experienced periods of drought and high rainfall. In the years 1788–1860 south-eastern Australia experienced 27 years of drought and New South Wales, 14 years of heavy rain [99]. The variability of rainfall in Australia, among the world’s highest, demonstrates in wet and dry periods often with extreme transition periods between the two [55]. The “Big Dry” from 1997–2009 was extreme and record breaking in its length [100]. The resultant research highlighted this weather event as a combination of traditional solar variability amplified by anthropogenic global warming from increased atmospheric greenhouse gases [54, 56]. Extremes in weather, including decreased rainfall, increased maximum temperatures, flash and basin flooding will impact on wildlife with greater risk of fires, decreased ground cover and possible increased erosion and sediment loading within catchments, rivers and lakes [55].
Chronic stress: impacts on reproduction and immune system
Disruptions to the environment and activation of the hypothalamic-pituitary-adrenal (HPA) axis (Fig. 1) generally prepares the body for some form of exertion ([9], [8, 101]). The hypothalamus releases corticotrophin-releasing hormone (CRH) [7], signalling the anterior pituitary to release adrenocorticotrophic hormone (ACTH) [8], which circulates in the blood and results in an increased output of glucocorticoids from the adrenal cortices [101]. Glucocorticoids act to divert storage of glucose, as glycogen or fat, and to mobilise glucose from stored glycogen. Cortisol is the pivotal glucocorticoid within HPA-axis stimulation and then restoration of homeostasis following the physiological stress response [101, 102]. Cortisol stimulates gluconeogenesis, preparing the animal for physical challenge, by partitioning energy, it also assists in balancing pH after the challenge, and finally acts as a chemical blocker, within a negative feedback process [102], to CRH secretion and HPA axis synergy. This interplay between hormonal and metabolic responses are similar across multiple vertebrate taxa and crucial for the maintenance of homeostasis [9, 10]. The HPA axis function comes at a cost of diverting energy away from other corporal functions [3, 7, 8]. Many authors reference concomitant reduction in growth, reproduction [48] and immune function [9, 66, 102] associated with chronic stress.
Chronic stress negatively impacts reproduction. At the levels of the neuroendocrine systems, each component of the HPA axis acts as an inhibitor to the hypothalamic-pituitary-gonadal (HPG) axis and once the HPA axis is activated, secretion of gonadotropin-hormone releasing hormone (GnRH) [8] and pituitary gonadotrophin responsiveness is lowered; testis and ovarian sensitivity to gonadal hormones become reduced [7]. Suppression of the reproductive function during an acute stress episode should be ephemeral whilst priorities of energy expenditure are shifted towards survival rather than reproduction [8] however, chronic stress and continued high levels of glucocorticoids prolong the inhibitory effect on reproduction and may also increase testicular apoptosis [12, 13] contributing to decreased circulating testosterone levels [12]. Aerobic mitochondrial metabolism as a consequence generates reactive oxygen species (ROS) [8, 48] and in the absence of pro-oxidant-antioxidant balance oxidative stress ensues [103]. Leydig cell apoptosis as a result of increased glucocorticoid release generates ROS, which may compromise sperm integrity and fetal development [104, 105].
We hypothesise that wild koala populations are most likely impacted by chronic stressors (diseases and anthropogenic induced environmental change) and they could also be undergoing sub-clinical changes to their reproductive system, such as suppression of gonadal functions, irregular reproductive hormone cycles and infertility in both males and females. These postulations require urgent investigation and it is recommended that general health checks of wild rescued koalas should also include physiological stress evaluation and reproductive health assessment [106, 107].
The stress endocrine response, despite many negative connotations, is a physiological function integral to survival [3, 108] and evidence suggest that vertebrates are designed to display plasticity in response to environmental challenges and evolutionary pressures to maintain allostasis ([3, 9, 15, 18]). Dhabar et al. [109] in studies using rats demonstrated that the immune system response to acute stress is adaptive. The acute stress response resulted in redistribution of immune cells; norepinephrine (NE)/epinephrine (EPI) facilitated an increase in immune cells within the blood stream and EPI/corticosterone (CORT) caused procession of immune cells from the blood stream to the skin [18], lymphoid tissue and to other sites involved in ‘de novo immune activation’ [109]. In acute stress, fleeing from a predator, interspecies aggression or the hunt for food there is a presumption that the immune response would have adapted to a biological system which is energy efficient, expediently activated and which would protect the animal from skin trauma, bites or non-specific infection, natural corollaries of an exertive effort. This system would also allow for energy to be redistributed to systems engaged in the physical effort. Moller and Saino [82] demonstrated a significant correlation between survival and strong innate immune responses in birds conferring an obvious fitness advantage over those with compromised immune system. Conversely, in the Dhabhar & McEwen [18] study delayed type hypersensitivity (DTH) was lessened and skin leukocytes were attenuated under chronic stress, which correlated with decreased response to glucocorticoids. The implications of a suppressed DTH in combination with attenuated skin lymphocytes may decrease resistance to opportunistic or harmful pathogens [18]. A temporary increase in Natural Killer cells (NKC) followed by suppression in response to peripheral β-adrenergic activation has been demonstrated in rats [110–113] and is part of the innate immune system, however Shakhar & Ben-Eliyahu [114] demonstrated that continuous acute levels of catecholamine were retrograde to NKC sensitive tumour line. The authors comment that a differentiation should be drawn between stress events and major sympathetic activation in which a dysfunction of NKC activity may allow metastasis to establish. Forced swimming [110] and attacked/submissive intruders [112] increased lung tumour metastasis retention/colonisation in rats whilst Vegas et al. [113] did not investigate NKC but found mice with poor social coping strategies, including low social exploration, defensiveness, low social position or avoidance developed more pulmonary metastasis. NKC are considered part of the innate immune system [115] and following challenge with tumour cell inoculation, retention and development of metastasis, are rigidly controlled for 24 h by NKC [112]. The discovery, in mice, of NK cells showing an adaptive immune response, antigen memory and specificity, in the absence of T & B cells [116] to a ‘hapten based contact sensitiser’ [115] is interesting within the chronic stress-immune system dyad. Social isolation stress, in mice challenged with bacterial endotoxin, has been shown to increase arginine vasopressin (AVP), decrease CRH mRNA (glucocorticoid receptor) and increases endotoxic shock susceptibility [17]. The biological impacts of environmental pollutants on koala populations have not been studied in great detail. Thus, it will be important to investigate the potential impacts of chemical pollutants such as atrazine and other agricultural chemicals as it has been shown that environmental pollutants have the capacity to increase infectious disease susceptibility through chronic stress and of the immune and reproductive system dysfunction (see [15]).
Diseases
Whilst loss of habitat, predation, climate change and fire have been widely studied as important environmental stressors there has been very little research into the decline of marsupial species resultant of toxic chemical exposure [117, 118]. Cluster disease events such as birth defects/thalidomide [119], employees occupationally exposed to polyvinyl chloride/liver angiosarcoma [120], typhoid outbreaks/water quality [121], childhood leukaemia associated/polluted well water [122], fluorosis in kangaroos/aluminium smelter [123, 124] and environmental/animal/human contamination due to polybrominated biphenyls in Michigan [125] indicate that some disease clusters are signals of environmental exposure [126], the consequence of which may have been preventable [127]. Bridging the gap between epidemiological studies, which identify environmental contamination as the probable cause of a disease manifestation and providing regulators with scientific certainties to enact policy often appears at juxtaposition with mitigation of the disease cluster; obvious xenobiotic exposure dismissed as insignificant [125, 127].
Several clusters of disease affect Australian wildlife; oxalate nephrosis in the South Australia koala [128, 129], chlamydia in Queensland koala [39, 130], Tasmanian devil facial tumor [131, 132], mycotic diseases in amphibians [133], mucormycosis in the Tasmanian platypus [134], two of these within a small biome, and increasing amounts of retrovirus, causing leukaemia and lymphoma [135] many not seen before 1950 indicating a commonality of environmental stressor. Rhodes et al. [136] considers that mortality from diseases must be reduced by 59 % to prevent further koala declines. Therefore, identification of environmental stressors that can lead to a decrease in the immune system function is critical to koala conservation and recovery programs.
Oxalate nephrosis
Data from 225 koalas from South Australia represented in Fig. 2 clearly demonstrates oxalate nephrosis (ON) as the predominant disease condition affecting koalas; 26.2 % of the koalas admitted to the hospital diagnosed (November 2013-December 2014) and is considered the leading disease within the Mt Lofty ranges, east of Adelaide in South Australia [128, 129]. This condition, although also present in the Queensland and New South Wales koala is considered of limited incidence and impact in those states [128]. Estimates of 11 % renal dysfunction Mt Lofty Ranges populations in 2000 [128] and 55 % in 2014 demonstrating gross or histopathological changes with oxalate crystal deposition [129] consistent with ON shows a rapid temporal progression [129, 137]. Azotaemia (higher than normal blood urea-BUN) in combination with poorly concentrated urine and low specific gravity was associated with increased severity of histopathological changes [128, 129], which may point to renal disease as the ultimate cause within a complex patho-physiological dynamics.
Clinical cases of koalas (N = 225) presented for treatment at a wildlife rescue facility in South Australia from November 2013–December 2014. Many patients presented with multiple conditions reflected in the tabled number of diagnosed condition versus patient numbers. Clearly in SA the major challenge for koalas is oxalate nephrosis, heat stress, car impacts and particularly for males dog attack. Unknown and trauma cases are well represented, many indicative of injuries sustained during dog attack or car collision and had these been included a more profound effect would have been shown. C = confirmed; U = unconfirmed
It is worthwhile to note that metatherian embryos (e.g. koalas) compared to eutherian embryos (e.g. chicken) require significant development dependent upon maternal milk during pouch development. Notably, marsupial mothers dedicate greater investment in lactation than in gestation [138]. Therefore, the effect of nutritional restriction [139–141] and increased maternal circulating glucocorticoids [139, 142–144] on the fetus during critical windows of development can result in decreased nephron numbers [139–141, 143, 144] predisposing offspring to continued nephron loss, progressive renal dysfunction/injury [139], glomerulosclerosis [145] and enhance sensitivity to secondary postnatal environmental insult [139]. Low glomerular number is an important determinant of renal function [146]. Speight et al. [128] found glomerular atrophy in all koala patients with ON accompanied by fibrosis associated with crystal obstruction of the tubules. This may point to a duality of causal factors; maternal stress and nutritional deprivation impacting foetal development in combination with and proximate environmental stressor/s acting on already compromised kidney function. Calcium phosphate, generally associated with calcium oxalate crystals, was found in both healthy and ON koalas [128] and may be only functionally important when renal function is already compromised. The prevalence of ON in young koalas [137] may preclude over consumption of oxalate as a cause implicating a pre-existing renal developmental disorder. The similar incidence of ON in captive koala populations [128] is suggestive of reasons other than a maternal gestational nutritional restriction impacting fetal kidney development pointing to stress and increased maternal glucocorticoids as the primary cause in both captive & wild populations. Alanine-glyoxylate aminotransferase (AGT) is an enzyme expressed in the hepatocytes and it is crucial for the metabolism of glyoxylate, the end product oxalate, excreted in urine [147]. Isolated populations of decreased genetic diversity show an autosomal disorder demonstrated by AGT deficiency [128, 137, 147, 148] however a decrease in AGT activity was not shown in SA koalas affected by ON [137]. Mt Lofty eucalypts showed higher levels of oxalates than Queensland species but despite this dietary oxalic acid ingestion was not considered as the cause of ON [128] however the results were pooled which may not allow for koala feeding exclusivity on eucalypts with the high oxalic acid content of some eucalypt species.
Koalas show a region/species preference [24, 149] with SA koalas displaying a marked preference for E. viminalis (manna gum) [150] and although koalas prefer to browse on young leaves in the wild, they are more likely to consume mature leaves due to availability. Manna gum and messmate leaf samples in the Mt Lofty ranges showed high oxalic acid concentration (4.68, 5.22 and 7.51 % DW respectively) with mature leaves overall showing higher oxalic acid content [128] during Spring sampling. Eucalypt species during times of low rainfall make osmoric adjustments, which reduces leaf water content [151]. Drought stress has been described in the Eucalypt with messmate stringy bark most affected [152]; peach trees demonstrate decreased leaf water content and increased oxalic acid levels [153]. Sampling of the same trees during summer or on the fringe may have had demonstrated different oxalic acid levels and the suggestion would be that eucalyptus seasonal, area specific oxalic acid levels be investigated with koala preferences adjusted for.
Other causal factors for ON are ingestion of ethylene gycol [129], which appears unlikely, however the extensive use of ethylene glycol in airplane deicers, automotive industry and solvents (ethylene glycol, http://www.npi.gov.au/resource/ethylene-glycol-12-ethanediol) and the proximity of ON cases to the Adelaide airport (Fig. 3) requires further investigation. Statistics Australia wide show alimentary tract and intestinal disease represented in 40 and 35 % of the koalas respectively. The possibility exists that over absorption of oxalic acid due to intestinal disease [154], decreased oxalic acid specific anaerobic bacteria [129, 154, 155] or slow microbial adaptation [156] is a factor in the development of ON disease and also requires further investigation.
Habitual factors mediate the composition of intestinal microbiota however the absence of bacteria with anti-inflammatory action during disease may implicate the essential nature of a functional intestinal bacterial flora for health [157, 158] and further investigation on the effects of elevated glucocorticoids on koala intestinal bacteria is required. Treatment indicated for ON is increased hydration [148], considered quite problematic with low leaf moisture content for the koala. Vickneswaran et al. [144] found male rat nephron numbers were most affected during gestation by maternal stress however Speight et al. [128] found no evidence of koala gender involvement in ON. The identification that prenatal nephron deficiency can be corrected with a single dose of retinoic acid [159] during gestation, even with nutritional deprivation, and during lactation [141] shows system plasticity, which can be exploited, particularly if environmental stressors can be identified and mitigated.
Chlamydiosis
Koalas suffer from two types of chlamydiosis, Chlamydia pneumoniae [39, 130], which may be endemic within koalas and C. pecorum, responsible for substantial reproductive consequences for the species [38, 39, 160] and possibly result of cross-species transmission [28]. Evidence suggests the chlamydia is associated with nutritional/environmental stressors [27] and symptoms vary from conjunctivitis, which may lead to blindness, respiratory tract infections [130], reproductive discharge and possible infertility [38].
The identification of genes from the natural killer complex (NKC) and leukocyte receptor complex (LRC) in koalas with chlamydia is suggestive of a role in the immune system [38] and may implicate the down-regulation of NKC activity during periods of chronic stress within individual manifestations and severity of chlamydial disease [40]. Rhodes et al. [136], Melzer et al. [27], and Phillips [28] question the impact chlamydiosis has on reproductive rates or survival of the koala with the possibility that ‘stable coexistence’ [27, 28] between disease and the koala has been demonstrated in population modelling [28]. Rhodes et al. [136] cites high rate of mortality rather than reproductive suppression in coastal koala populations as the stressor limiting the growth rate of the population. Chlamydia diagnosis, in the presence of an inflamed cloaca, without diagnostic confirmation or despite negative results (Narayan unpublished data) may have over emphasised the incidence of the disease within koala populations.
Fringe effects
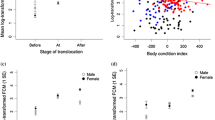
High cortisol levels in koalas are associated with range edge, lower rainfall and leaf moisture [161] with continuously high cortisol levels, indicating chronic stress, for example, in a fragmented spider monkey colony [162]. Within dry or high temperature environments, leaf moisture dictates eucalyptus choice [27, 163] and combined with fur insulation, low basal metabolism, decreased body temperature [164], evaporative loss from the respiratory tract [165] and the ability to utilise non-fodder trees where the daytime temperature is lower [27, 166] allow thermoregulation and arboreal existence. Ellis et al. [165] found non-fodder daytime trees favoured by koalas were 2 °C cooler than the ambient temperature and are a critical feature of the koala microclimate. During drought and heatwaves koala survival depends upon relocation to a riparian habitat [24, 161, 165, 167, 168] and the availability of free standing water [161] without which dehydration follows. Contraction of riparian habitat is associated with mass mortality events in young koalas pushed from moist sites to the fringe [167] and expectation for fragmented or fringe populations in extreme temperature events increases extinction risk [161, 168]. Heat stress cases, reflected in Fig. 4, clearly show an association between koala heat stress, suburban fringe and temperatures exceeding 40 °C, the exception being 4 heat stress cases at 28.3 °C, however the preceding day was 44.7 °C and demonstrates the potential for heat stress, possibly resultant of dehydration, despite cooler temperatures [169].
Heat stress is a major environmental stressor in the koala. Anthropogenic driven global warming with an increase in extreme temperature events pose a substantial risk of mortality when koala colonies have limited access to riparian habitats. It shows the number of heat stress cases against environmental temperature; cases are strongly associated with days of high temperature. Four cases were recorded for a moderate temperature of 28.3 °C however the preceding day was 44.7 °C and illustrates the limited ability of the koala to recover from extreme temperatures despite moderation of conditions. Temperature data was obtained from the Bureau of Meteorology (http://www.bom.gov.au/)
Higher leaf moisture is associated with soil water holding capacity [161]; the assumption being that soil would be moisture deprived at the fringe of a habitat. The koala, a caecum-colon fermener [170] maintains a precarious energy balance [171, 172], utilising poor quality diets containing essential oils and tannins within an extensive, prolonged, microbial digestive process [173]. The basal metabolism of the koala is 74 % of the mean of other marsupials [173] with maintenance energy requirements similarly reflected [164, 173]. Koalas spend around 19.3 – 20 h a day resting or sleeping [172, 174] however hypervigilance has been demonstrated in response to human presence/noise [171]. The energetic cost of chronic stress impacts reproduction [12, 13], growth [7], and the immune system [7, 15] whilst hypervigilance, the relationship with proximity to suburbia (Fig. 3) creates an energy/water/thermoregulation deficit [171, 172] when unable to engage in physiological and behavioural adaptations [174]. Large/small body size, within a Bergman’s rule (smaller body size as environmental heat increases) creates a paradox for the koala within degraded, fragmented/fringe habitats with water deprived environments experiencing extreme temperature events resultant of anthropogenic climate warming [5, 24, 54, 56, 57, 175]. Small body size in Queensland koalas allows for effective heat dissipation [176] but in the absence of water may encourage dehydration [5]. Koalas are larger in temperate areas as males size under sexual selection [176, 177] and as a result need more water [172] but would consequently have an inability to dissipate heat. This is clearly reflected in heat stress cases (Figs. 3 and 4) in which males (N = 17) and females (N = 11) showed marked difference in SA koalas.
Predation and road trauma
Rhodes et al. [136] and McAlpine et al. [31] commented that as forest cover is reduced mortality events increase; koala population decline considered to be driven by increased mortality events [31]. Species within a fragmented or isolated habitat have a low probability of survival [178–180]. According to Davies et al. [25, 26], koalas living in the edge of its range often increase their movement in search of food sources and increase their vulnerability to predation in doing so. Both rural and suburban fragmentation/fringe effects are high risk for dog attack/vehicle trauma [28, 31, 35] with natal dispersal of 2–3 year old males clearly represented within deaths (Fig. 5). Absence of overlapping canopy encourages koalas to cross gaps between trees on the ground increasing vulnerability to dog-attack and vehicle trauma [31]. Reduced abundance of vertebrates worldwide is associated with proximity to roads [181]; road trauma, noise [181] and pollution must be also considered within koala chronic stress dynamics.
Conclusions
Our literature review provides fresh evidence that koala populations in urban and fringe areas are being severely impacted by environmental stressors. It is the synergistic nature of environmental stressors that makes the impact much more severe and the prolonged nature of stressors, arrival of new stressors (such as new urban development projects) are causing increasingly more stress to koala populations. We have identified key areas of investigation for future research, including a stronger focus on diseases (e.g. maternal transfer of immune genes to developing embryos), impacts on reproductive fitness traits (e.g. sperm integrity and mortality) and stronger research focus under the thematic area of chronic stress. Clearly, the field of conservation physiology, through non-invasive reproductive and stress hormone monitoring technologies have an important position in koala conservation and management research programs [107, 182]. Koala recovery programs should also focus on the potential impacts of environmental pollutants on the populations, especially those living in close proximity to urban zones. Native wildlife species can provide real-life examples of how anthropogenic induced environmental changes are impacting on Australia’s once pristine terrestrial environments. Hence, we should start thinking more seriously about our actions and how we are impacting nature. Stronger support is needed for research and conservation management programs to save our native fauna from the threat of extinction.
Abbreviations
ACTH, adrenocorticotrophic hormone; AGT, alanine-glyoxylate aminotransferase; AVP, arginine vasopressin; BUN, blood urea; CORT, corticosterone; CRH, corticotrophin-releasing hormone; DTH, delayed type hypersensitivity; EPI, epinephrine; GnRH, gonadotropin-hormone releasing hormone; HPA, hypothalamic-pituitary-adrenal; HPG, hypothalamic-pituitary-gonadal; KoRV, koala retrovirus; LRC, leukocyte receptor complex; NE, norepinephrine; NKC, natural killer cells; ON, oxalate nephrosis; ROS, reactive oxygen species
References
Hughes L. Climate change and Australia: trends, projections and impacts. Aust Ecol. 2003;28:423–43.
Van der Ree R, McCarthy MA. Inferring persistence of indigenous mammals in response to urbanisation. Anim Conserv. 2005;8(03):309–19.
Wingfield JC. The comparative biology of environmental stress: behavioural endocrinology and variation in ability to cope with novel changing environments. Anim Behav. 2013;85(5):1127–33.
Woinarski JCZ, Burbidge AA, Harrison PL. Ongoing unraveling of a continental fauna: Decline and extinction of Australian mammals since European settlement. Proceedings of the National Academy of Sciences. doi:10.1073/pnas.1417301112 - See more at: http://www.smh.com.au/national/gone-feral-the-cats-devouring-our-wildlife-20140911-10fbs1.html 2015
McKechnie AE, Wolf BO. Climate change increases the likelihood of catastrophic avian mortality events during extreme heat waves. Biol Lett. 2010;6:253–6. doi:10.1098/rsbl.2009.0702.
Taylor DL, Leung LP, Gordon IJ. The impact of feral pigs (Sus scrofa) on an Australian lowland tropical rainforest. Wildl Res. 2011;38(5):437–45.
O’Connor TM, O’Halloran DJ, Shanahan F. The stress response and the hypothalamic‐pituitary‐adrenal axis: from molecule to melancholia. QJM. 2000;93(6):323–33.
Whirledge S, Cidlowski JA. Glucocorticoids, stress, and fertility. Minerva Endocrinol. 2010;35(2):109.
Hing S, Narayan E, Thompson RA, Godfrey S. A review of factors influencing the stress response in Australian marsupials. Conserv Physiol. 2014;2(1):cou027.
Dantzer B, Fletcher QE, Boonstra R, Sheriff MJ. Measures of physiological stress: a transparent or opaque window into the status, management and conservation of species? Conserv Physiol. 2014;2(1):cou023.
Herman JP. Neural control of chronic stress adaptation. Front Behav Nuerosci. 2013;7:1–12.
Gao HB, Tong MH, Guo QS, Ge R, Hardy MP. Glucocorticoid induces apoptosis in rat leydig cells. Endocrinology. 2002;143(1):130–8.
Yazawa H, Sasagawa I, Nakada T. Apoptosis of testicular germ cells induced by exogenous glucocorticoid in rats. Hum Reprod. 2000;15(9):1917–20.
Narayan E, Jessop T, Hero J-M. Invasive cane toad triggers chronic physiological stress and decreased reproductive success in an island endemic. Funct Ecol. 2015;29(11):1435–44.
Acevedo-Whitehouse K, Duffus ALJ. Effects of environmental change on wildlife health. Philos Trans R Soc B. 2009;364(1534):3429–38.
Marshall Jr GD, Agarwal SK, Lloyd C, Cohen L, Henninger EM, Morris GJ. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav Immun. 1998;12(4):297–307.
Quan N, Avitsur R, Stark JL, He L, Shah M, Caligiuri M, Padgett DA, Marucha PPT, Sherida J. Social stress increases the susceptibility to endotoxic shock. J Neuroimmunol. 2001;115(1):36–45.
Dhabhar FS, McEwen BS. Acute stress enhances while chronic stress suppresses cell mediated immunity in vivo: a potential role for leukocyte trafficking. Brain Behav Immun. 1997;11(4):286–306.
Amery-Gale J, Vaz PK, Whiteley P, Tatarczuch L, Taggart DA, Charles JA, Wilks CR. Detection and Identification of a Gammaherpesvirus in Antechinus spp. in Australia. J Wildl Dis. 2014;50(2):334–9.
Arthur AD, Catling PC, Reid A. Relative influence of habitat structure, species interactions and rainfall on the post‐fire population dynamics of ground‐dwelling vertebrates. Aust Ecol. 2012;37(8):958–70.
Eymann J, Smythe LD, Symonds ML, Dohnt MF, Barnett LJ, Cooper DW, Herbert CA. Leptospirosis serology in the common brushtail possum (Trichosurus vulpecula) from urban Sydney, Australia. J Wildl Dis. 2007;43(3):492–7.
McCallum H, Jones M, Hawkins C, Hamede R, Lachish S, Sinn DL, Lazenby B. Transmission dynamics of Tasmanian devil facial tumor disease may lead to disease-induced extinction. Ecology. 2009; 90(12):3379–3392
Holderness-Roddam B, McQuillan PB. Domestic dogs (Canis familiaris) as a predator and disturbance agent of wildlife in Tasmania. Aust J Environ Manag. 2014;21(4):441–52.
Black KH, Price GJ, Archer M, Hand SJ. Bearing up well? Understanding the past, present and future of Australia’s koalas. Gondwana Res. 2014;25(3):1186–201.
Davies N, Gramotnev G, Seabrook L, Bradley A, Baxter G, Rhodes J, Lunney D, McAlpine C. Movement patterns of an arboreal marsupial at the edge of its range: a case study of the koala. Mov Ecol. 2013;1(1):8.
Davies NA, Gramotnev G, McAlpine C, Seabrook L, Baxter G, Lunney D, Rhodes JR, Bradley A. Physiological stress in Koala populations near the arid edge of their distribution. PLoS ONE. 2013;8(11):e79136.
Melzer A, Carrick F, Menkhorst P, Lunney D, John BS. Overview, critical assessment, and conservation implications of koala distribution and abundance. Conserv Biol. 2000;14(3):619–28.
Phillips SS. Population trends and the koala conservation debate. Conserv Biol. 2000;14(3):650–9.
Tsangaras K, Ávila-Arcos MC, Ishida Y, Helgen KM, Roca AL, Greenwood AD. Historically low mitochondrial DNA diversity in koalas (Phascolarctos cinereus). BMC Genet. 2012;13(1):92.
Vaz P, Whiteley PL, Wilks CR, Duignan PJ, Ficorilli N, Gilkerson JR, Devlin JM. Detection of a novel gammaherpesvirus in koalas (Phascolarctos cinereus). J Wildl Dis. 2011;47(3):787–91.
McAlpine CA, Rhodes JR, Callaghan JG, Bowen ME, Lunney D, Mitchell DL, Pullar DV, Possingham HP. The importance of forest area and configuration relative to local habitat factors for conserving mammals: a case study of koalas in Queensland, Australia. Biol Conserv. 2006;132(2):153–65.
Davies N, Gramotnev G, Seabrook L, McAlpine C, Baxter G, Lunney D, Bradley A. Climate-driven changes in diet composition and physiological stress in an arboreal folivore at the semi-arid edge of its distribution. Biol Conserv. 2014;172:80–8.
Johansen MP, Twining JR. Radionuclide concentration ratios in Australian terrestrial wildlife and livestock: data compilation and analysis. Radiat Environ Biophys. 2010;49(4):603–11.
Liu C, Deng J, Yu L, Zhou B. Endocrine disruption and reproductive impairment in Zebrafish by exposure to 8:2 fluorotelomer alcohol. Aquat Toxicol. 2010;96(1):70–6.
Brearley G, Rhodes J, Bradley A, Baxter G, Seabrook L, Lunney D, Liu Y, McAlpine C. Wildlife disease prevalence in human-modified landscapes. Biol Rev. 2013;88(2):427–42.
Buddle BM, Young LJ. Immunobiology of mycobacterial infections in marsupials. Dev Comp Immunol. 2000;24(5):517–29.
Fyfe JA, Lavender CJ, Handasyde KA, Legione AR, O’Brien CR, Stinear TP, McCowan C. A major role for mammals in the ecology of Mycobacterium ulcerans. PLoS Negl Trop Dis. 2010;4(8):e791.
Morris KM, Mathew M, Waugh C, Ujvari B, Timms P, Polkinghorne A, Belov K. Identification, characterisation and expression analysis of natural killer receptor genes in Chlamydia pecorum infected koalas (Phascolarctos cinereus). BMC Genomics. 2015;16(1):1.
Polkinghorne A, Hanger J, Timms P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet Microbiol. 2013;165(3):214–23.
Mathew M, Beagley KW, Timms P, Polkinghorne A. Preliminary characterisation of tumor necrosis factor alpha and interleukin-10 responses to Chlamydia pecorum infection in the koala (Phascolarctos cinereus). Plos One. 2013;8(3):e59958.
Denner J, Young PR. Koala retroviruses: characterization and impact on the life of koalas. Retrovirology. 2013;10(1):108.
Houlden BA, England PR, Taylor AC, Greville WD, Sherwin WB. Low genetic variability of the koala Phascolarctos cinereus in south‐eastern Australia following a severe population bottleneck. Mol Ecol. 1996;5(2):269–81.
Daszak P, Cunningham AA, Hyatt AD. Anthropogenic environmental change and the emergence of infectious diseases in wildlife. Acta Trop. 2001;78(2):103–16.
Hoque A. Risk of spill-over of diseases (in particular avian influenza) from wild aquatic birds in North Queensland. 2011. PhD thesis, James Cook University.
Plowright RK, Eby P, Hudson PJ, Smith IL, Westcott,D, Bryden WL, Tabor GM. Ecological dynamics of emerging bat virus spillover. Proc R Soc Lond B Biol Sci. 2015; 282(1798):20142124.
Gober P. Desert urbanization and the challenges of water sustainability. Curr Opin Environ Sustain. 2010;2:144–50.
Burbridge AA, McKenzie NL. Patterns in the modern decline of Western Australia’s vertebrate fauna. Biol Conserv. 1989;50(1):143–98.
Beaulieu M, Costantini D. Biomarkers of oxidative status: missing tools in conservation physiology. Conserv Physiol. 2014;2(1):1–16.
Hickling R, Roy DB, Hill JK, Fox R, Thomas CD. The distribution of a wide range of taxonomic groups are expanding polewards. Glob Chang Biol. 2006;12(3):450–5.
Rosenzweig C, Karoly D, Vicarelli M, Neofotis P, Wu Q, Casassa G, Menzel A, Root TL, Estrella N, Seguin B, Tryjanowski P, Liu C, Rawlins S, Imeson A. Attributing physical and biological impacts to anthropogenic climate change. Nature. 2008;453(7193):353–7.
Walther G-R, Post E, Convey P, Menzel A, Parmesan C, Beebee TJC, Fromentin J-M, Hoegh-Goldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416(6879):389–95.
Plant R, Walker J, Rayburg S, Gothe J, Leung T. The wild life of pesticides: urban agriculture, institutional responsibility, and the future of biodiversity in Sydney’s Hawksbury Nepean River. Aust Geogr. 2012;43(1):75–91.
Wood MD, Beresford NA, Semenov DV, Yankovich TL, Copplestone D. Radionuclide transfer to reptiles. Radiat Environ Biophys. 2010;49(4):509–31.
Karoly DJ, Braganza K. Attribution of recent temperature changes in the Australian region. Am Meteorol Soc. 2005;18(3):457–61. 463–464.
Pittock B, Abbs D, Suppiah R, Jones R. Climatic background to past and future floods in Australia. Adv Ecol Res. 2006;39:13–39.
Timbal B, Arblaster J, Braganza K, Fernandez E, Hendon H, Murphy B, Raupach M, Smith I, Whan K, Wheeler M. Understanding the anthropogenic nature of the observed rainfall decline across south-east Australia. The Centre for Australian Weather and Climate Research (CAWR). 2010. Technical report No, 026, 202 pp. Downloaded from: http://www.cawcr.gov.au/technical-reports/CTR_026.pdf on 10-2-2016.
Welbergen JA, Klose SM, Markus N, Eby P. Climate change and the effects of temperature extremes on Australian flying-foxes. Proc R Soc Lond B. 2008;275(1633):419425.
Facelli JM, Temby AM. Multiple effects of shrubs on annual plant communities in arid lands of South Australia. Aust Ecol. 2002;27(4):422–32.
Glen AS, Dickman CR. Niche overlap between marsupial and eutherian carnivores: does competition threaten the endangered spotted‐tailed quoll? J Appl Ecol. 2008;45(2):700–7.
Johnson CN, Isaac JL, Fisher DO. Rarity of a top predator triggers continent-wide collapse of mammal prey: dingoes and marsupials in Australia. Proc R Soc Lond B Biol Sci. 2007;274(1608):341–6.
Fancourt BA, Hawkins CE, Cameron EZ, Jones ME, Nicol SC. Devil declines and catastrophic cascades: is mesopredator release of feral cats inhibiting recovery of the eastern quoll? PLoS ONE. 2015;10(3):e0119303.
Glen AS, Pennay M, Dickman CR, Wintle BA, Firestone KB. Diets of sympatric native and introduced carnivores in the Barrington Tops, eastern Australia. Aust Ecol. 2011;36(3):290–6.
Kennedy M, Phillips BL, Legge S, Murphy SA, Faulkner RA. Do dingoes suppress the activity of feral cats in northern Australia? Aust Ecol. 2015;63(4):258–69.
Haythorpe K. Competitive behaviour in Common Mynas. An investigation into potential impacts on native fauna and solutions for management. 2014. PhD thesis, Charles Sturt University.
Kaňuščák P, Hromada M, Tryjanowski P, Sparks T. Does climate at different scales influence the phenology and phenotype of the River Warbler Locustella fluviatilis? Oecologia. 2004;141(1):158–63.
Narayan EJ. Evaluation of physiological stress in Australian wildlife: embracing pioneering and current knowledge as a guide to future research directions. General and Comparative Endocrinology. in-press. Accepted 10/12/15.
Stahl T, Mattern D, Brunn H. Toxicology of perfluorinated compounds. Environ Sci Eur. 2011;23(1):1–52.
Adam-Guillerman C, Pereira S, Delia-Vedova C, Hinton T, Garnier-Laplace J. Genotoxic and reprotoxic effects of tritium and external gamma irradiation on aquatic animals. In: Whitacre DM, editor. Reviews of environmental contamination and toxicology. 2012. p. 67–101. 220. Springer Science + Business Media, LLC.
Ankley GT, Bencic DC, Breen MS, Collette TW, Conolly RB, Denslow ND, Edwards SW, Ekman DR, Garcia-Reyero N, Jensen KM, Lazorchak JM, Martinovic D, Miller DH, Perkins EJ, Orlando EF, Villeneuve DL, Wang R-L, Watanabe KH. Endocrine disrupting chemicals in fish: developing exposure indicators. Aquat Toxicol. 2009;92(3):168–78.
Bony S, Gaillard I, Devaux A. Genotoxicity assessment of two vineyard pesticides in zebrafish. Int J Environ Anal Chem. 2010;90(3–6):421–8.
Buckley J, Willingham E, Agras K, Baskin LS. Embryonic exposure to the fungicide vinclozolin causes virilation of females and alteration of progesterone receptor expression in vivo: an experimental study in mice. Environ Health. 2006;5(4):1–6.
Gagnaire B, Adam-Guillermin C, Bouron A, Lestaevel P. The effects of radionuclides on animal behaviour. In: Whitacre DM, editor. Reviews of environmental contamination and toxicology. 2011. p. 35–58. 210. Springer Science + Business Media, LLC.
Goetz AK, Dix DJ. Mode of action for reproductive and hepatic toxicity inferred from genomic study of Triazole antifungals. Toxicol Sci. 2009;110(2):449–62.
Alford RA, Bradfield KS, Richards SJ. Ecology: global warming and amphibian losses. Nature. 2007;447(7144):E3–4.
Ryan U, Power M. Cryptosporidium species in Australian wildlife and domestic animals. Parasitology. 2012;139(13):1673–88.
Thompson RCA, Lymbery AJ, Smith A. Parasites, emerging disease and wildlife conservation. Int J Parasitol. 2010;40(10):1163–70.
Vermeulen ET, Ashworth DL, Eldridge MDB, Power ML. Investigation into potential transmission sources of Giardia duodenalis in a threatened marsupial (Petrogale penicillata). Infect Genet Evol. 2015;33:277–80.
Appelbee AJ, Thompson RCA, Olson ME. Giardia and Cryptosporidium in mammalian wildlife-current status and future needs. Trends Parasitol. 2005;21(8):370–6.
Gelis S, Spratt DM, Raidal SR. Neuroangiostrongyliasis and other parasites in tawny frogmouths (Podargus strigoides) in south‐eastern Queensland. Aust Vet J. 2011;89(1–2):47–50.
Jenkins DJ. Hydatid control in Australia: where it began, what we have achieved and where to from here. Int J Parasitol. 2005;35(7):733–40.
Lidetu D, Hutchinson GW. The prevalence, organ distribution and fertility of cystic echinoccosis in feral pigs in tropical North Queensland, Australia. Onderstepoort J Vet Res. 2007;74(1):73–9.
Moller AP, Saino N. Immune response and survival. OIKOS. 2004;104(2):299–304.
Thompson J, Yang R, Power M, Hufschmid J, Beveridge I, Reid S, Ng J, Armson A, Ryan U. Identification of zoonotic Giardia genotypes in marsupials in Australia. Exp Parasitol. 2008;120(1):88–93.
Berry O, Kirkwood R. Measuring recruitment in an invasive species to determine eradication potential. J Wildl Manag. 2010;74(8):1661–70.
Courchamp F, Chapuis JL, Pascal M. Mammal invaders on islands: impact, control and control impact. Biol Rev. 2003;78(03):347–83.
Medina FM, Bonnaud E, Vidal E, Nogales M. Underlying impacts of invasive cats on islands: not only a question of predation. Biodivers Conserv. 2014;23(2):327–42.
Vine S, Dutson G. (2006–2009). IBAs in Danger: The state of Australia’s important bird and biodiversity areas. Birdlife. Accessed from: http://birdlife.org.au/documents/IBA-IBAs-in-danger-Nov14.PDF on 17-2-2016 at 12.00PM
Harrington G, Freeman A, Murphy S, Venables B, Edwards C. Carpentarian Grasswren survey. Prepared for the Queensland Department of Environment and Resource Management by Birds Australia North Queensland. Downloaded from: http://birdlifenq.org/pdfs/boodjamulla_2011_final_report.pdf on 16–2 at 1:19PM. 2011
Norman JA, Christidis L. Ecological opportunity and the evolution of habitat preferences in the arid-zone bird: implications for speciation in a climate-modified landscape. Sci Rep. 2016;6(19613):1–12.
Serventy VN, Pringle JD, Lindsey TR. The wrens and warblers of Australia. Sydney: Angus& Robertson; 1982.
Van Der Wal J, Murphy HT, Kutt AS, Pperkins GC, Bateman BL, Perry JJ, Reside AE. Focus on poleward shifts in species’ distribution underestimates the fingerprint of climate change. Nat Clim Chang. 2013;3(3):239–43.
Gienapp P, Teplitsky C, Alho JS, Mills JA, Merilä J. Climate change and evolution: disentangling environmental and genetic responses. Mol Ecol. 2008;17(1):167–78.
Gardner JL, Heinsohn R, Joseph L. Shifting latitudinal clines in avian body size correlate with global warming in Australian passerines. Proc R Soc B. 2009;276(1674):3845–38452.
Gasc A, Duryea MC, Cox RM, Kern A, Calsbeek R. Invasive predators deplete genetic diversity of island lizards. PLoS ONE. 2010;5(8):e12061.
Boersma PD, Rebstock GA. Climate change increases reproductive failure in Magellanic penguins. Plos one. 2014;9(1):e85602.
Loarie SR, Duffy PB, Hamilton H, Asner GP, Field CB, Ackerly DD. The velocity of climate change. Nature. 2009;462(7276):1052–7.
Chen I-C, Hill JK, Ohlemiller R, Roy DB, Thomas CD. Rapid change shifts species associated with high levels of climate warming. Science. 2011;333(6045):1024–6.
Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421(6918):37–42.
Fenby C, Gergis J. Rainfall variations in south-eastern Australia part 1: consolidating evidence from pre-instrumental documentary sources, 1788–186. 2012.
Gergis J, Ashcroft L. Rainfall variations in south-eastern Australia part 2: a comparison of documentary, early instrumental and palaeoclimate records, 1788–2008. 2012.
Möstl E, Palme R. Hormones as indicators of stress. Domest Anim Endocrinol. 2002;23(1):67–74.
Romero LM. Physiological stress in ecology: lessons from biomedical research. Trends Ecol Evol. 2004;19(5):249–55.
Sies H. Oxidative stress: from basic research to clinical application. Am J Med. 1991;30(61,3C):31S–8.
Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16(1):31–8.
Marchetti F, Bishop JB, Cosentino L, Moore II D, Wyrobek AJ. Paternally transmitted chromosomal aberrations in mouse zygotes determine their embryonic fate. Biol Reprod. 2004;70(3):616–24.
Ballantyne K, Lisle A, Mucci A, Johnston SD. Seasonal oestrous cycle activity of captive female koalas in south-east Queensland. Aust Mammal. 2015;37:245–52.
Narayan E, Webster K, Nicolson V, Hero J-M. Non-invasive evaluation of physiological stress in an iconic Australian marsupial: the Koala (Phascolarctos cinereus). Gen Comp Endocrinol. 2013;187:39–47.
Segerstrom SC, Miller GE. Psychological stress and the immune system: a meta-analysis study of 30 years on inquiry. Psychol Bull. 2004;130(4):601–30.
Dhabar FS, Malarkey WB, Neri E, McEwen BS. Stress-induced redistribution of immune cells-from barracks to boulevards to battlefields: a tale of three hormones-Curt Richter award winner. Psychoneuroendocrinology. 2012;37(9):1345–68.
Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK cell activity and of resistance to metastasis by stress: a role for adrenal catecholamines and beta-adrenoceptors. Neuroimmunomodulation. 2000;8(5):154–64.
Ben-Eliyahu S, Shakhar G, Page GG, Stefanski V, Shakhar K. Suppression of NK Cell Activity and of Resistance to Metastasis by Stress: A role for Adrenal Catecholamines and β-Adrenoceptors. Neuroimmunomodulation. 2000;8:154–64. doi:10.1159/000054276.
Stefanski V, Ben-Eliyahu S. Social confrontation and tumor metastasis in rats: defeat and β-Adrenergic mechanisms. Physiol Behav. 1996;60(1):277–82.
Vegas O, Fano E, Brain PF, Alonso A, Azpiroz A. Social stress, coping strategies and tumour development in male mice: behavioural, neuroendocrine and immunological implications. Psychoneuroendocrinology. 2006;31(1):69–79.
Shakhar G, Ben-Eliyahu S. In vivo β-adrenergic stimulation suppresses natural killer activity and compromises resistance to tumor metastasis in rats. J Immunol. 1998;160(7):3251–8.
Paust S, Senman B, Von Andrian UH. Adaptive immune responses mediated by natural killer cells. Immunol Rev. 2010;235(1):286–96.
O’Leary JG, Goodarzi M, Drayton DI, von Adrian UH. T cell- and B cell-independent adaptive immunity mediated by natural cell killer cells. Nat Immunol. 2006;7(5):507–16.
Bolton RM, Ahokas JT. REVIEW: detoxication in Australian marsupials-ecotoxicological implications. Australas J Ecotoxicol. 1995;1:85–98.
Stupans I, Kong S, Kirlich A, Murray M, Bailey EL, Jones BR, McKinnon RA. Hepatic microsomal enzyme activity in the koala and tammar wallaby: high 17β-hydroxysteroid oxidoreductase activity in koala liver microsomes. Comp Biochem Physiol C: Pharmacol Toxicol Endocrinol. 1999;123(1):67–73.
Vargesson N. Thalidomide‐induced limb defects: resolving a 50‐year‐old puzzle. Bioessays. 2009;31(12):1327–36.
Creech Jr JL, Johnson MN. Angiosarcoma of liver in the manufacture of polyvinyl chloride. J Occup Environ Med. 1974;16(3):150–2.
Ram PK, Naheed A, Brooks WA, Hossain MA, Mintz ED, Breiman RF, Luby SP. Risk factors for typhoid fever in a slum in Dhaka, Bangladesh. Epidemiol Infect. 2007;135(3):458–65.
Lagakos SW, Wessen BJ, Zelen M. An analysis of contaminated well water and health effects in Woburn, Massachusetts. J Am Stat Assoc. 1986;81(395):583–96.
Clarke E, Beveridge I, Slocombe R, Coulson G. Fluorosis as a probable cause of chronic lameness in free ranging eastern grey kangaroos (Macropus giganteus). J Zoo Wildl Med. 2006;37(4):477–86.
Kierdorf U, Death C, Hufschmid J, Witzel C, Kierdorf H. Developmental and post-eruptive defects in molar enamel of free-ranging eastern grey kangaroos (macropus giganteus) exposed to high environmental levels of fluoride. PLoS ONE. 2016;11(2):e0147427.
Reich MR. Environmental politics and science: the case of PBB contamination in Michigan. Am J Public Health. 1983;73(3):302–13.
Aamodt G, Samuelsen SO, Skrondal A. A simulation study of three methods for detecting disease clusters. Int J Health Geogr. 2006;5(1):1.
Clapp R, Howe G, LeFevre M. Environmental and occupational causes of cancer: review of recent scientific literature. The Lowell Center for Sustainable Production, University of Massachusetts Lowell. 2005.
Speight KN, Breed WG, Boardman W, Taggart DA, Leigh C, Rich B, Haynes JI. Leaf oxalate content of Eucalyptus spp. and its implications for koalas (Phascolarctos cinereus) with oxalate nephrosis. Aust J Zool. 2013;61(5):366–71.
Speight KN, Haynes JI, Boardman W, Breed WG, Taggart DA, Rich B, Woolford L. Plasma biochemistry and urinalysis variables of koalas (Phascolarctos cinereus) with and without oxalate nephrosis. Vet Clin Pathol. 2014;43(2):244–54.
Marsh J, Kollipara A, Timms P, Polkinghorne A. Novel molecular markers of Chlamydia pecorum genetic diversity in the koala (Phascolarctos cinereus). BMC Microbiol. 2011;11(1):1.
Cheng Y, Fox S, Pemberton D, Hogg C, Papenfuss AT, Belov K. The Tasmanian devil microbiome—implications for conservation and management. Microbiome. 2015;3(1):1.
Ross T. Persistent chemicals in Tasmanian devils, Independent scientist report commissioned by the Save The Tasmanian Devil Program, Tasmania. 2008.
Densmore CL, Green DE. Diseases of amphibians. Ilar J. 2007;48(3):235–54.
Grant TR, Temple-Smith PD. Conservation of the platypus, Ornithorhynchus anatinus: threats and challenges. Aquat Ecosyst Health Manag. 2003;6(1):5–18.
Simmons G, Clarke D, McKee J, Young P, Meers J. Discovery of a novel retrovirus sequence in an Australian native rodent (Melomys burtoni): a putative link between gibbon ape leukemia virus and koala retrovirus. PLoS ONE. 2014;9(9):e106954.
Rhodes JR, Ng CF, de Villiers DL, Preece HJ, McAlpine CA, Possingham HP. Using integrated population modelling to quantify the implications of multiple threatening processes for a rapidly declining population. Biol Conserv. 2011;144(3):1081–8.
Speight KN. Oxalate nephrosis in a population of South Australian koalas (Phascolarctos cinereus). 2013.
Jones CB, 2012 Robustness, Plasticity, and Evolvability in Mammals. Mammals: From Humble Vertebrate Beginnings to Global Terrestrial Dominance. Part of the series SpringerBriefs in Evolutionary Biology. p 7–20. doi:10.1007/978-1-4614-3885-4_2.
Paixão AD, Alexander BT. How the kidney is impacted by the perinatal maternal environment to develop hypertension. Biol Reprod. 2013;89(6):144.
Symonds ME, Stephenson T, Gardner DS, Budge H. Long-term effects of nutritional programming of the embryo and fetus: mechanisms and critical windows. Reprod Fertil Dev. 2007;19:53–63.
Wlodek ME, Mibus A, Tan A, Siebel AL, Owens JA, Moritz KM. Normal lactational environment restores nephron endowment and prevents hypertension after placental restriction in the rat. J Am Soc Nephrol. 2007;18(6):1688–96.
Dickinson H, Walker DW, Wintour EM, Moritz K. Maternal dexamethasone treatment at midgestation reduces nephron number and alters renal gene expression in the fetal spiny mouse. Am J Phys Regul Integr Comp Phys. 2007;292(1):R453–61.
O’Sullivan L, Cuffe JS, Paravicini TM, Campbell S, Dickinson H, Singh RR, Moritz KM. Prenatal exposure to dexamethasone in the mouse alters cardiac growth patterns and increases pulse pressure in aged male offspring. PLoS ONE. 2013; 8(7): e69149.
Vickneswaran C, Jefferies AJ, Anevska K, Cheong JN, Hanvey A, Singh RR, Wlodek ME. Maternal stress during pregnancy programs nephron deficits and gender specific hypertension in second generation offspring. In: Hypertension (Vol. 63, No. 6). 2014. p. E141. Lippincott Williams and Wilkins.
Luyckx VA, Shukha K, Brenner BM. Low nephron number and its clinical consequences. Rambam Maimonides Med J. 2011;2(4):e0061. doi:10.5041/RMMJ.10061.
Hoy WE, Bertram JF, Denton RD, Zimanyi M, Samuel T, Hughson MD. Nephron number, glomerular volume, renal disease and hypertension. Curr Opin Nephrol Hypertens. 2008;17(3):258–65.
Pey AL, Albert A, Salido E. Protein homeostasis defects of alanine-glyoxylate aminotransferase: new therapeutic strategies in primary hyperoxaluria type I. BioMed Res Int. 2013;2013(687658):15. http://dx.doi.org/10.1155/2013/687658.
Cochat P, Liutkus A, Fargue S, Basmaison O, Ranchin B, Rolland MO. Primary hyperoxaluria type 1: still challenging! Pediatr Nephrol. 2006;21(8):1075–81.
Phillips S, Callaghan J, Thompson V. The tree species preferences of koalas (Phascolarctos cinereus) inhabiting forest and woodland communities on Quaternary deposits in the Port Stephens area, New South Wales. Wildl Res. 2000;27(1):1–10.
Ullrey DE, Robinson PT, Whetter PA. Composition of Preferred and Rejected Eucalyptus Browse Offered to Captive Koalas, Phascolarctos Cinereus (Marsupialia). Aust J Zool. 1981;29(6):839–46.
Merchant A, Callister A, Arndt S, Tausz M, Adams M. Contrasting physiological responses of six Eucalyptus species to water deficit. Ann Bot. 2007;100(7):1507–15.
Sinclair R. Water potential and stomatal conductance of three Eucalyptus species in the Mount Lofty Ranges, South Australia: responses to summer drought. Aust J Bot. 1980;28(6):499–510.
Arndt SK, Wanek W, Clifford SC, Popp M. Contrasting adaptations to drought stress in field-grown Ziziphus mauritiana and Prunus persica trees: water relations, osmotic adjustment and carbon isotope composition. Funct Plant Biol. 2000;27(11):985–96.
Allison MJ, Cook HM, Milne DB, Gallagher S, Clayman RV. Oxalate degradation by gastrointestinal bacteria from humans. J Nutr. 1986;116(3):455–60.
Abratt VR, Reid SJ. Oxalate-degrading bacteria of the human gut as probiotics in the management of kidney stone disease. Adv Appl Microbiol. 2010;72:63–87.
Allison MJ, Littledike ET, James LF. Changes in ruminal oxalate degradation rates associated with adaptation to oxalate ingestion. J Anim Sci. 1977;45(5):1173–9.
Penders J, Thijs C, van den Brandt PA, Kummeling I, Snijders B, Stelma F, Stobberingh EE. Gut microbiota composition and development of atopic manifestations in infancy: the Koala Birth Cohort Study. Gut. 2007;56(5):661–7
Round JL, Mazmanian SK. The gut microbiota shapes intestinal immune responses during health and disease. Nat Rev Immunol. 2009;9(5):313–23.
Makrakis J, Zimanyi MA, Black MJ. Retinoic acid enhances nephron endowment in rats exposed to maternal protein restriction. Pediatr Nephrol. 2007;22(11):1861–7.
Mathew M, Pavasovic A, Prentis PJ, Beagley KW, Timms P, Polkinghorne A. Molecular characterisation and expression analysis of Interferon gamma in response to natural Chlamydia infection in the koala, Phascolarctos cinereus. Gene. 2013;527(2):570–7.
Davies N, Gillett A, McAlpine C, Seabrook L, Baxter G, Lunney D, Bradley A. The effect of ACTH upon faecal glucocorticoid excretion in the koala. J Endocrinol. 2013;219(1):1–12.
Rangel‐Negrín A, Alfaro JL, Valdez RA, Romano MC, Serio‐Silva JC. Stress in Yucatan spider monkeys: effects of environmental conditions on fecal cortisol levels in wild and captive populations. Anim Conserv. 2009;12(5):496–502.
Munks SA, Corkrey R, Foley WJ. Characteristics of arboreal marsupial habitat in the semi-arid woodlands of Northern Queensland. Wildl Res. 1996;23(2):185–95.
Degabriele R, Dawson TJ. Metabolism and heat balance in an arboreal marsupial, the koala (Phascolarctos cinereus). J Compa Physiol B: Biochem Syst Environ Physiol. 1979;134(4):293–301.
Ellis W, Melzer A, Clifton I, Carrick F. Climate change and the koala Phascolarctos cinereus: water and energy. Aust Zool. 2010;35(2):369–77.
de Oliveira SM, Murray PJ, de Villiers DL, Baxter GS. Ecology and movement of urban koalas adjacent to linear infrastructure in coastal south-east Queensland. Aust Mammal. 2014;36(1):45–54.
Gordon G, Brown AS, Pulsford T. A koala (Phascolarctos cinereus Goldfuss) population crash during drought and heatwave conditions in south‐western Queensland. Aust J Ecol. 1988;13(4):451–61.
Seabrook L, McAlpine C, Baxter G, Rhodes J, Bradley A, Lunney D. Drought-driven change in wildlife distribution and numbers: a case study of koalas in south west Queensland. Wildl Res. 2011;38(6):509–24.
Adelaide temperatures. The Bureau of Meteorology. Accessed from: http://www.bom.gov.au/jsp/ncc/cdio/weatherData/av?p_nccObsCode=122&p_display_type=dailyDataFile&p_startYear=2015&p_c=−106708561&p_stn_num=023090 on the 15-3-2016 at 10: 50AM.
Cork SJ, Hume ID. Microbial digestion in the koala (Phascolarctos cinereus, Marsupialia), an arboreal folivore. J Comp Physiol. 1983;152(1):131–5.
Larsen MJ, Sherwen SL, Rault JL. Number of nearby visitors and noise level affect vigilance in captive koalas. Appl Anim Behav Sci. 2014;154:76–82.
Nagy KA, Martin RW. Field metabolic rate, water flux, food consumption and time budget of koalas, phascolarctos cinereus (marsupialia: phascolarctidae) in Victoria. Aust J Zool. 1985;33(5):655–65.
Cork SJ, Hume ID, Dawson TJ. Digestion and metabolism of a natural foliar diet (Eucalyptus punctata) by an arboreal marsupial, the koala (Phascolarctos cinereus). J Comp Physiol. 1983;153(2):181–90.
Benesch AR, Munro U, Krop T, Fleissner G. Seasonal changes in the behaviour and circadian rhythms in activity and behaviour of captive koalas Phascolarctos cinereus. Biol Rhythm Res. 2010;41(4):289–304.
McKechnie AE, Hockey PA, Wolf BO. Feeling the heat: Australian landbirds and climate change. Emu-Austral Ornithol. 2012;112(2):i.
Briscoe NJ, Krockenberger A, Handasyde KA, Kearney MR. Bergmann meets Scholander: geographical variation in body size and insulation in the koala is related to climate. J Biogeogr. 2015;42(4):791–802.
Charlton BD, Ellis WAH, Brumm J, Nilsson K, Tecumseh Fitch W. Female koalas prefer bellows in which lower formants indicate larger males. Anim Behav. 2012;84(6):1565–71.
Lindenmayer DB, Lacy RC. Small mammals, habitat patches and PVA models: a field test of model predictive ability. Biol Conserv. 2002;103(3):247–65.
Santos-Filho M, Peres CA, Da Silva DJ, Sanaiotti TM. Habitat patch and matrix effects on small-mammal persistence in Amazonian forest fragments. Biodivers Conserv. 2012;21(4):1127–47.
Yates MD, Loeb SC, David Jr C. The effect of habitat patch size on small mammal populations. 1997.
van der Ree R, Jaeger JAG, van der Grift EA, Clevenger AP. Effects of roads and traffic on wildlife populations and landscape function: road ecology is moving towards larger scales. Ecology and Society. 2011;16(1):48. [online] URL: http://www.ecologyandsociety.org/vol16/iss1/art48/.
Narayan E. Non-invasive reproductive and stress endocrinology in amphibian conservation physiology. Conservation Physiology. 2013;1:1–16.
Acknowledgements
We wish to wholeheartedly thank the veterinary staff, nurses and volunteers of the Adelaide Koala and Wildlife Hospital (AKWH). Special thanks to Sue Finch, Dr Phil Hutt, Dr Sheridan Lathe for their generous support.
Availability of data and materials
This is a review and all data presented has been previously published and citations have been provided. The Adelaide Koala and Wildlife Hospital has been duly acknowledged for providing clinical data that have been adequately presented in graphs. Raw data may be requested through consent of the AKWH.
Authors’ contributions
EN conceptualised the review paper and the Masters of Science Research Scholar (MW) conducted the literature collection and data collation. Both authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics approval and consent to participate
Ethics approval was not required for review article. Dr Narayan holds Charles Sturt University Animal Care and Ethics protocol (#A10644) for collaborative koala research with the Adelaide Koala and Wildlife Hospital.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated.
About this article
Cite this article
Narayan, E.J., Williams, M. Understanding the dynamics of physiological impacts of environmental stressors on Australian marsupials, focus on the koala (Phascolarctos cinereus). BMC Zool 1, 2 (2016). https://doi.org/10.1186/s40850-016-0004-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40850-016-0004-8