Abstract
Background
Although small bowel obstruction (SBO) is a major complication occurring after abdominal surgery, few reports have described strangulated SBO after pelvic lymphadenectomy (PL). This report describes two cases of strangulated SBO caused by a skeletonized obturator nerve and pelvic vessels after laparoscopic PL during gynecologic surgery.
Case presentation
Case 1: A 57-year-old woman with endometrial cancer underwent a laparoscopic semi-radical total hysterectomy with PL. Nine months after the operation, she visited our emergency room complaining about subacute pain spreading in the right groin, right buttock, and dorsal part of the right thigh. She had no abdominal pain. Although her symptoms were not typical, computed tomography (CT) revealed strangulated SBO in the right pelvis. Laparoscopic surgery revealed that the small bowel was ischemic. Then we converted to open surgery. We transected the right obturator nerve and umbilical artery, which constructed an internal hernia orifice in the right pelvis, followed by resection of the ischemic small bowel. Fortunately, during 6-month follow-up, she showed only slight difficulty in walking as a postoperative complication. Case 2: A 62-year-old woman with cervical cancer underwent laparoscopic radical hysterectomy with PL. Six months after the operation, she visited our hospital emergently because of sudden onset of abdominal pain and vomiting. CT showed strangulated SBO. Urgent laparoscopic surgery exhibited the incarcerated small bowel at the right pelvis. Consequently, we converted to open surgery. The terminal ileum was detained into the space constructed by the right umbilical artery. We cut the umbilical artery and performed ileocecal resection. After the surgery, she was discharged with no complication or sequela.
Conclusion
When examining a patient after PL who complains of severe pain or symptoms, one should consider the possibility of PL-related SBO, even if the pain is apparently atypical for SBO.
Similar content being viewed by others
Background
Pelvic lymphadenectomy (PL) is intended as a complete cure of multiple malignant diseases in the pelvic cavity [1,2,3,4,5,6]. Recently, minimally invasive surgery using laparoscopic and robot-assisted modalities have also been applied to this surgical procedure [7,8,9,10]. Strangulated small bowel obstruction (SBO) induced by exposed vessels and nerves in the pelvic cavity is a rare complication after PL. Rapid diagnosis is crucially important. Nevertheless, diagnosing them accurately is much more challenging if they present atypical symptoms. This report describes two rare cases of PL-related strangulated SBO after laparoscopic PL. One patient showed typical symptoms and radiological findings as incarcerated SBO, whereas the other displayed atypical symptoms such as severe pain in the dorsal part of the thigh with no abdominal pain, which was difficult to diagnose accurately as strangulated SBO.
Case presentation
Case 1
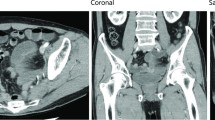
A 57-year-old woman (body mass index, 18.9 kg/m2) visited our emergency room for the chief complaint of subacute pain spreading in the right groin, right buttock, and dorsal part of the right thigh. Regarding the abdominal findings, she showed no tenderness or distention. Nine months prior, she had undergone a laparoscopic semi-radical total hysterectomy with bilateral salpingo-oophorectomy, pelvic lymphadenectomy, para-aortic lymphadenectomy, partial omentectomy, and peritoneal stripping for endometrial cancer. After surgery, she received standard systemic adjuvant chemotherapy for 6 months. No recurrent sign was observed. When she visited our hospital, we first suspected sciatica from her chief complaint. Computed tomography (CT) images of the abdomen and pelvis were obtained to ascertain the causes of her chief complaint. Unexpectedly, CT images revealed that small bowel obstruction (SBO) without a closed-loop had occurred in the right pelvic wall (Fig. 1a and b). At this time point, however, her pain had completely disappeared because of the administration of an analgesic. She therefore reported no abdominal pain. The laboratory data indicated moderate inflammation, WBC 10,110/μL, and lactate level 28.8 mg/dL. These clinical data and symptoms were atypical and insufficient to diagnose her as having strangulated SBO. Therefore, we chose hospitalization to facilitate close follow-up of her general condition. Six hours after hospitalization, she became affected again by acute, similar severe pain in the dorsal part of the right thigh. Follow-up CT presented more edematous mesentery of the small bowel and an increase of ascites (Fig. 1c and d), strongly indicating that the strangulated SBO was worsening.
Abdominal CT scan images of the case 1 patient taken at her first visit (a, b) and 6 h after hospitalization (c, d). a and b Coronal (a) and axial (b) enhanced CT scan images at her first visit showed dilated small intestine without a closed loop. c and d Coronal (c) and axial (d) plain CT scan images 6 h after the hospitalization exhibited edematous mesentery (yellow circle) and a closed loop, the origin of which was in the right pelvic wall (yellow arrowhead)
Therefore, we performed emergent laparoscopic surgery and found the strangulated small bowel with bloody ascites in the right pelvis. The strangulated small bowel appeared to be severely ischemic (Fig. 2a). The dilated oral side of the small bowel prevented us from grasping it with laparoscopic forceps safely and from making a clear surgical view. Therefore, we converted to open surgery to confirm the cause of the strangulated ileus. Results showed two isolated bands in the right pelvic region, which caused the internal hernia orifice of the strangulated small bowel. We were unable to help transecting both to release the strangulated small bowel. The incarcerated small bowel was finally resected because the blood flow did not recover. After resection of the incarcerated small bowel, it became clear that the bands constructing the internal hernia orifice were the right obturator nerve and umbilical artery (Fig. 2b). Although the initial gynecologic surgery had been highly invasive, none of the small bowel adhered to itself or to the abdominal walls. Adhesion-preventing material had been used before completing that operation. The patient progressed favorably after the operation. She was discharged with no major complication on the 6th postoperative day. Presently, she has right adductor muscle weakness (manual muscle test: MMT 4/5), but showed only slight walking difficulties at 6-month follow-up.
Case 2
A 62-year-old woman (body mass index, 20.6 kg/m2) visited our hospital emergently because of sudden onset of abdominal pain and vomiting. Six months prior, the patient had undergone laparoscopic radical hysterectomy with bilateral salpingo-oophorectomy and pelvic lymphadenectomy for cervical cancer and showed no recurrence signs. Laboratory data exhibited marked elevation of the white blood cell count (15,760/μL) and the lactate level (49.5 mg/dL). Enhanced abdominal CT scan demonstrated massive ascites, edematous mesentery, and ischemic small bowel near the right pelvic wall, strongly suggesting the possibility of strangulated SBO (Fig. 3a–c). Immediately after starting urgent laparoscopic surgery, massive bloody ascites and incarcerated small bowel were observed (Fig. 3d). As in case 1, the small intestine did not adhere to itself or to the abdominal wall. Adhesion-preventing material had also been used in gynecologic surgery. The incarcerated small bowel was severely dilated and was apparently ischemic ultimately. Therefore, after converting to open surgery, we found the terminal ileum detained into the space constructed by the right umbilical artery. The right umbilical artery was resected to extract the ischemic small bowel. Ileocecal resection was performed. She was discharged with no important complication on the 7th postoperative day.
Abdominal CT scan images before urgent surgery (a–c) and a laparoscopic view after resection of umbilical artery (d) of the case 2 patient. a Massive ascites are found on the liver surface. b and c Axial (b) and coronal view (c) of the strangulated small bowel. The yellow arrowhead and circle denote the strangulated origin and edematous mesentery and intestine. d Umbilical artery stump (white arrowhead) after resection of the incarcerated small bowel and a cord
Discussion
We experienced two cases of strangulated SBO after PL during gynecologic surgery. In both cases, emergent operations were necessary, with cutting of the bands constructing the internal hernia orifice and resecting the incarcerated small bowel. The bands in the first case were the right umbilical artery and obturator nerve, whereas the other was the right umbilical artery. Ascertaining the cause of the strangulated SBO clearly before the operations was challenging. Especially in the first case, the patient displayed atypical symptoms such as severe pain in her right buttock with no abdominal pain. Retrospectively, we inferred the pain she exhibited as similar to Howship–Romberg sign in a patient with an obturator hernia [11].
PL is a standard surgical procedure performed for several malignant diseases affecting the pelvic organs, such as ovarian [1], cervical, endometrial [2, 3], prostate [4], bladder [5], and rectal cancers [6]. Strangulated internal hernia involving the right common iliac artery after PL in a patient with testicular cancer was first reported in 1978 [12]. No report for 30 years thereafter described strangulated internal hernia related to skeletonized vessels or nerves after PL. In 2008, Kim et al. reported strangulated internal hernia involving the right external iliac artery in a patient with cervical cancer [13]. To date, a total of 19 cases have been described in 17 reports, including ours (Table 1). We assume that the recent increase of case reports related to PL-related SBO might be attributable to the development of adhesion-preventing materials [14,15,16] and to the wider utilization of minimally invasive surgery such as laparoscopic and robot-assisted surgery [7,8,9,10].
It is particularly interesting that about half of reports have originated from the gynecologic field, in which all the initial gynecologic surgeries were done laparoscopically [13, 17,18,19,20,21,22,23]; moreover, approximately one-third of those reports were from the urologic area, in which the primary operations, except for the first case reported, were laparoscopic or robot-assisted surgeries [12, 24,25,26,27,28]. Recently, two reports described three cases after rectal cancer surgeries [29, 30]. In general, PL performed in gynecologic and urological fields can dissect lymph nodes around an external iliac artery or common iliac artery [1, 2, 4]. However, these lymphadenectomies are not always done in rectal cancer [6]. Perhaps for that reason, among others, few related reports from the rectal cancer field have been published.
According to past papers, vessels or nerves constructing the internal hernia orifice were right common iliac artery (3 cases) [12, 18, 24], left external iliac artery and/or vein (4 cases) [17, 22, 25, 27], right external iliac artery and/or vein (4 cases) [13, 20, 23, 26], right superior vesical artery (3 cases) [21, 29] and right umbilical artery and/or obturator nerve (5 cases) [19, 28, 30]. In fact, PL-related SBO is more common on the right side than on the left side (Table 1), which might be attributable to the fact that the left side is covered with the sigmoid colon.
The median time to onset from PL was 6 months, but its distribution was from 2 to 108 months, underscoring the point that PL-related SBO can occur anytime in patients with a history of PL. Three of four cases in which the incarcerated small bowel was preserved were of laparoscopic techniques [17, 19, 22, 23], whereas open surgeries were performed in 13 of 15 cases in which the incarcerated small bowel was removed, including five cases converted from laparoscopic surgeries. Those findings might reflect the difficulty, in many cases, of providing the patients with an accurate diagnosis rapidly.
The patient in case 1 presented symptoms similar to the Howship–Romberg sign. Generally, there are relationships between the symptoms of obturator hernia and the thin body; however, previous reports did not refer to any connections between PL-related SBO and the lean body.
Other than our cases, only three published reports describe PL-related SBO caused by a band constructed by the obturator nerve [19, 28, 30]. In these cases, the authors preserved the obturator nerve, leaving the hernia orifice unrepaired. By contrast, we resected the obturator nerve in our case. Generally, damage to the obturator nerve can induce leg weakness and gait disorders. Some patients also present sensory symptoms or severe pain in the groin, buttock, and medial thigh [31, 32]. Ningshu et al. described that severe damage to the obturator nerve causes permanent neurological deficits and motor weakness [33]. At the same time, they described the possibility of using analgesics, physiotherapy, and obturator nerve blockade for obturator neuropathy. In fact, the patient in case 1 complained of right adductor muscle weakness (MMT 4/5) immediately after surgery, but it had almost disappeared at 6-month follow-up. Therefore, even if resection of the obturator nerve is unavoidable, conservative management should be considered to recover or alleviate symptoms after the operation.
Conclusions
When examining a patient after PL who complains of severe pain or symptoms, we should consider the possibility of PL-related SBO, even if the pain is apparently atypical for SBO.
Availability of data and materials
Datasets supporting the conclusions of this article are included within the article.
Abbreviations
- SBO:
-
Small bowel obstruction
- PL:
-
Pelvic lymphadenectomy
- CT:
-
Computed tomography
- MMT:
-
Manual muscle test
References
Yoshihara M, Kajiyama H, Tamauchi S, Iyoshi S, Yokoi A, Suzuki S, et al. Prognostic impact of pelvic and para-aortic lymphadenectomy on clinically-apparent stage I primary mucinous epithelial ovarian carcinoma: a multi-institutional study with propensity score-weighted analysis. Jpn J Clin Oncol. 2020;50(2):145–51.
Aslan K, Meydanli MM, Oz M, Tohma YA, Haberal A, Ayhan A. The prognostic value of lymph node ratio in stage IIIC cervical cancer patients triaged to primary treatment by radical hysterectomy with systematic pelvic and para-aortic lymphadenectomy. J Gynecol Oncol. 2020;31(1): e1.
Morice P, Castaigne D, Pautier P, Rey A, Haie-Meder C, Leblanc M, et al. Interest of pelvic and paraaortic lymphadenectomy in patients with stage IB and II cervical carcinoma. Gynecol Oncol. 1999;73(1):106–10.
García-Perdomo HA, Correa-Ochoa JJ, Contreras-García R, Daneshmand S. Effectiveness of extended pelvic lymphadenectomy in the survival of prostate cancer: a systematic review and meta-analysis. Cent Eur J Urol. 2018;71(3):262–9.
Li R, Petros FG, Davis JW. Extended pelvic lymph node dissection in bladder cancer. J Endourol. 2018;32(S1):S49-s54.
Atef Y, Koedam TW, van Oostendorp SE, Bonjer HJ, Wijsmuller AR, Tuynman JB. Lateral pelvic lymph node metastases in rectal cancer: a systematic review. World J Surg. 2019;43(12):3198–206.
Yuh B, Artibani W, Heidenreich A, Kimm S, Menon M, Novara G, et al. The role of robot-assisted radical prostatectomy and pelvic lymph node dissection in the management of high-risk prostate cancer: a systematic review. Eur Urol. 2014;65(5):918–27.
Bae SU, Saklani AP, Hur H, Min BS, Baik SH, Lee KY, et al. Robotic and laparoscopic pelvic lymph node dissection for rectal cancer: short-term outcomes of 21 consecutive series. Ann Surg Treat Res. 2014;86(2):76–82.
Kagawa H, Kinugasa Y, Shiomi A, Yamaguchi T, Tsukamoto S, Tomioka H, et al. Robotic-assisted lateral lymph node dissection for lower rectal cancer: short-term outcomes in 50 consecutive patients. Surg Endosc. 2015;29(4):995–1000.
Song SH, Choi GS, Kim HJ, Park JS, Park SY, Lee SM, et al. Long-term clinical outcomes of total mesorectal excision and selective lateral pelvic lymph node dissection for advanced low rectal cancer: a comparative study of a robotic versus laparoscopic approach. Tech Coloproctol. 2021;25(4):413–23.
Practical remarks on the discrimination and appearances of surgical disease: With an appendix, containing the descriptive catalogue of the Author's Collection in Pathological Anatomy; and the Hunterian Oration for 1833. Med Chir Rev. 1841;35(69):1–26.
Guba AM Jr, Lough F, Collins GJ, Feaster M, Rich NM. Iatrogenic internal hernia involving the iliac artery. Ann Surg. 1978;188(1):49–52.
Kim KM, Kim CH, Cho MK, Jeong YY, Kim YH, Choi HS, et al. A strangulated internal hernia behind the external iliac artery after a laparoscopic pelvic lymphadenectomy. Surg Laparosc Endosc Percutan Tech. 2008;18(4):417–9.
Tsapanos VS, Stathopoulou LP, Papathanassopoulou VS, Tzingounis VA. The role of Seprafilm bioresorbable membrane in the prevention and therapy of endometrial synechiae. J Biomed Mater Res. 2002;63(1):10–4.
Robertson D, Lefebvre G. Adhesion prevention in gynaecological surgery. J Obstet Gynaecol Can. 2010;32(6):598–602.
Krill LS, Ueda SM, Gerardi M, Bristow RE. Analysis of postoperative complications associated with the use of anti-adhesion sodium hyaluronate-carboxymethylcellulose (HA-CMC) barrier after cytoreductive surgery for ovarian, fallopian tube and peritoneal cancers. Gynecol Oncol. 2011;120(2):220–3.
Dumont KA, Wexels JC. Laparoscopic management of a strangulated internal hernia underneath the left external iliac artery. Int J Surg Case Rep. 2013;4(11):1041–3.
Ardelt M, Dittmar Y, Scheuerlein H, Barthel E, Settmacher U. Post-operative internal hernia through an orifice underneath the right common iliac artery after Dargent’s operation. Hernia. 2014;18(6):907–9.
Minami H, Nagasaki T, Akiyoshi T, Konishi T, Fujimoto Y, Nagayama S, et al. Laparoscopic repair of bowel herniation into the space between the obturator nerve and the umbilical artery after pelvic lymphadenectomy for cervical cancer. Asian J Endosc Surg. 2018;11(4):409–12.
Frenzel F, Hollaender S, Fries P, Stroeder R, Stroeder J. Jejunal obstruction due to rare internal hernia between skeletonized external iliac artery and vein as late complication of laparoscopic hysterectomy with pelvic lymphadenectomy-case report and review of literature. Arch Gynecol Obstet. 2020;302(5):1075–80.
Ai W, Liang Z, Li F, Yu H. Internal hernia beneath superior vesical artery after pelvic lymphadenectomy for cervical cancer: a case report and literature review. BMC Surg. 2020;20(1):312.
Zhang Z, Hu G, Ye M, Zhang Y, Tao F. A strangulated internal hernia beneath the left external iliac artery after radical hysterectomy with laparoscopic pelvic lymphadenectomy: a case report and literature review. BMC Surg. 2021;21(1):273.
Hishikawa T, Oura S, Tomita M. Successful surgical intervention of strangulated ileus with a simple cut of the external iliac vein without vein reconstruction. Case Rep Gastroenterol. 2021;15(3):846–51.
Pridjian A, Myrick S, Zeltser I. Strangulated internal hernia behind the common iliac artery following pelvic lymph node dissection. Urology. 2015;86(5):e23–4.
Viktorin-Baier P, Randazzo M, Medugno C, John H. Internal hernia underneath an elongated external iliac artery: a complication after extended pelvic lymphadenectomy and robotic-assisted laparoscopic prostatectomy. Urol Case Rep. 2016;8:9–11.
Kambiz K, Lepis G, Khoury P. Internal hernia secondary to robotic assisted laparoscopic prostatectomy and extended pelvic lymphadenectomy with skeletonization of the external iliac artery. Urol Case Rep. 2018;21:47–9.
Ninomiya S, Amano S, Ogawa T, Ueda Y, Shiraishi N, Inomata M, et al. Internal hernia beneath the left external iliac artery after robotic-assisted laparoscopic prostatectomy with extended pelvic lymph node dissection: a case report. Surg Case Rep. 2019;5(1):49.
Kanno T, Otsuka K, Takahashi T, Somiya S, Ito K, Higashi Y, et al. Strangulated internal hernia beneath the obturator nerve after laparoscopic radical cystectomy with extended pelvic lymph node dissection. Urology. 2020;145:11–2.
Kitaguchi D, Nishizawa Y, Sasaki T, Tsukada Y, Kondo A, Hasegawa H, et al. A rare complication after laparoscopic lateral lymph node dissection for rectal cancer: two case reports of internal hernia below the superior vesical artery. J Anus Rectum Colon. 2018;2(3):110–4.
Uehara H, Yamazaki T, Kameyama H, Iwaya A, Gohda Y, Chinen I, et al. Internal hernia beneath the obturator nerve after robot-assisted lateral lymph node dissection for rectal cancer: a case report and literature review. Asian J Endosc Surg. 2020;13(4):578–81.
Bischoff C, Schönle PW. Obturator nerve injuries during intra-abdominal surgery. Clin Neurol Neurosurg. 1991;93(1):73–6.
Vasilev SA. Obturator nerve injury: a review of management options. Gynecol Oncol. 1994;53(2):152–5.
Ningshu L, Min Y, Xieqiao Y, Yuanqing Y, Xiaoqiang M, Rubing L. Laparoscopic management of obturator nerve schwannomas: experiences with 6 cases and review of the literature. Surg Laparosc Endosc Percutan Tech. 2012;22(2):143–7.
Acknowledgements
This work was supported by JSPS KAKENHI Grant Number 21K16487.
Funding
Japan Society for the Promotion of Science, 21K16487, Yoshihisa Okuchi.
Author information
Authors and Affiliations
Contributions
RI wrote the manuscript and prepared the figures and the table. YO, KK, and YI performed the surgeries. YO and KK reviewed and revised the manuscript. RM, KH, and KO supervised the editing of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from patients for publication of this case report and any accompanying images.
Competing interests
The authors declare that none has any competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ideyama, R., Okuchi, Y., Kawada, K. et al. Strangulated small bowel obstruction caused by isolated obturator nerve and pelvic vessels after pelvic lymphadenectomy in gynecologic surgery: two case reports. surg case rep 8, 104 (2022). https://doi.org/10.1186/s40792-022-01459-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-022-01459-w