Abstract
Background
Leiomyosarcoma is a rare tumor that could originate from the gastrointestinal tract, uterus, kidney, retroperitoneum, and the soft tissues of the extremities. It accounts for only 1% of all gastrointestinal mesenchymal tumors and primary leiomyosarcoma of the stomach is extremely rare. Most cases reported as leiomyosarcoma of the stomach before the development of KIT immunohistochemistry might be gastrointestinal stromal tumors (GISTs) of the stomach and only 18 cases of leiomyosarcoma of the stomach have been reported since early 2000s. We report here a patient with leiomyosarcoma of the stomach treated by laparoscopic and endoscopic cooperative surgery (LECS).
Case presentation
A 59-year-old man was referred to our hospital for an early gastric cancer, which was initially treated by endoscopic submucosal dissection. Six months after his initial treatment, a follow-up esophagogastroduodenoscopy revealed a small polypoid lesion at the lesser curvature of the proximal stomach, which appeared to be a hyperplastic polyp. However, one and a half years later, the lesion grew and showed more irregular surface. Biopsy at the time revealed smooth muscle cell proliferation suggestive of leiomyoma. Three years later, the lesion grew even larger and biopsy showed pleomorphic spindle cells. Immunohistochemical study showed positive staining for alpha-smooth muscle actin and desmin, but negative for c-kit and CD34. Ki-67 labeling index was nearly 60%. Based on these findings, the diagnosis of leiomyosarcoma was established. The patient subsequently underwent a partial gastrectomy by LECS. The patient is currently in good condition without recurrence or metastasis at 12 months after surgery.
Conclusions
Leiomyosarcoma of the stomach is extremely rare. This is the first report of leiomyosarcoma of the stomach treated by LECS. We could also follow its appearance change through endoscopic examination for 3 years.
Similar content being viewed by others
Background
Leiomyosarcoma is a rare tumor that could originate from the gastrointestinal tract, uterus, kidney, retroperitoneum, and the soft tissues of the extremities [1]. Primary leiomyosarcoma of the stomach is extremely rare, and most cases reported as leiomyosarcoma of the stomach before the development of KIT immunohistochemistry (pre-KIT era) might be gastrointestinal stromal tumors (GISTs) of the stomach [2]. To the best of our knowledge, only 18 patients with leiomyosarcoma of the stomach have been described in the literature. Currently, the standard treatment is surgical resection of the tumor [2]. Herein, we report a patient with extremely rare leiomyosarcoma of the stomach. We observed the growth of this tumor endoscopically for 3 years and treated the tumor with laparoscopic and endoscopic cooperative surgery (LECS). This is the first report of leiomyosarcoma of the stomach treated by LECS.
Case presentation
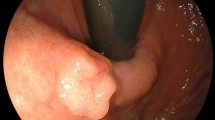
A 59-year-old male without significant chief complaints and medical history was referred to our hospital for treatment of early gastric adenocarcinoma. The patient underwent endoscopic submucosal dissection (ESD) to resect the lesion. Pathological examination demonstrated complete resection of the tumor. The resected tumor was confined within the mucosal layer without lymphovascular invasion. Six months after ESD, a follow-up esophagogastroduodenoscopy (EGD) was performed and found a small polypoid lesion at the lesser curvature of the proximal stomach, which appeared to be a benign polyp (Fig. 1a).
Subsequent EGD at 1 year after ESD, the polypoid lesion was clinically diagnosed as hyperplastic polyp based on its size (3 mm) and shape (Fig. 1b). One and a half years after ESD, the polypoid lesion grew in size and its surface became more irregular (Fig. 1c). However, biopsy did not show any signs of malignancy. Two years after initial ESD, biopsy showed smooth muscle cell proliferation without positive staining for the GISTs makers such as kit and CD34, which suggestive of leiomyoma (Fig. 1d). Three years after ESD, the lesion became even larger (8 mm in size) and its surface and vascular pattern became more irregular (Fig. 1e). Biopsy at this point revealed the tumor comprised pleomorphic spindle cells with noticeable atypia. Immunohistochemical study demonstrated positive staining for alpha-smooth muscle actin and desmin, but negative for c-kit, CD34, and AE1/AE3. Ki-67 labeling index was estimated as 60% and p16 was diffusely positive. Based on these findings, the tumor was diagnosed as leiomyosarcoma of the stomach. Computed tomography scans and fluorodeoxyglucose positron emission did not show any distant metastatic disease.
The patient subsequently underwent a partial gastrectomy by LECS. The location of the tumor was confirmed by intraoperative EGD, but was not identifiable with laparoscopy. The margin of the tumor was marked endoscopically by injecting both indigo carmine and hyaluronic acid into the submucosal layer (Fig. 2a). The gastric wall was dissected along the incision line from the laparoscopic side (Fig. 2b). The dissection line was clearly identified both laparoscopically and endoscopically. After the resection of the lesion, the incision was closed using hand-sewn sutures laparoscopically.
Intraoperative findings and resected specimen. The periphery of the tumor was marked by injecting indigo carmine and hyaluronic acid into the submucosal layer (a); the stomach was dissected along the marked incision line laparoscopically (b); macroscopically, the tumor measured 18 × 15 × 10 mm in size (c); relatively well-circumscribed, solid white tumor (d)
Macroscopically, the lesion was a well-circumscribed, solid white tumor and measured 18 × 15 × 10 mm in size (Fig. 2c, d). Histologically, hematoxylin and eosin staining revealed that the tumor was composed of atypical spindle cells with eosinophilic cytoplasm (Fig. 3a) derived from proper mucous membrane and submucosa (Fig. 3b). Immunohistochemistry demonstrated that the tumor cells were positive for alpha-smooth muscle antin (Fig. 3c) and desmin (Fig. 3d), but negative for CD34 (Fig. 3e) and S-100 protein, which was consistent with preoperative biopsy specimens. Ki-67 index was estimated to be 60% (Fig. 3f). Based on these findings, the diagnosis of leiomyosarcoma was confirmed. Post-operative course was uneventful and the patient was discharged on postoperative day 9. The patient underwent computed tomography scans every 6 months and EGD annually for postoperative surveillance. The patient also had blood work done every 3 months. The patient is currently in good condition without recurrence or metastasis at 12 months after surgery.
Pathological findings. Hematoxylin and eosin staining shows well-developed fascicles of atypical spindle cells with eosinophilic cytoplasm (a) (200×); derived from proper mucous membrane and submucosa (b) (100×). Immunohistochemistry showed the tumor cells were positive for alpha-smooth muscle actin (c) (200×), and desmin (d) (200×), and negative for CD 34 (e) (100×). Ki-67 index was estimated to be 60% (f) (100×)
Discussion
Leiomyosarcoma is a malignant mesenchymal tumor arising from smooth muscle cells [3]. Leiomyosarcoma of the gastrointestinal tract is a rare entity, which accounts for 1.1% of all gastrointestinal mesenchymal lesions [4]. Moreover, the stomach is the least common site for true leiomyosarcoma to originate [5]. Thus, gastric leiomyosarcoma is extremely rare. Leiomyosarcoma shows the pattern of intersecting marginated fascicles of spindle cells microscopically [6]. GISTs are also mesenchymal tumors with malignant potential [7]. GISTs show spindle cell pattern microscopically, which are believed to arise from the interstitial cells of Cajal or their precursors, located throughout the muscular wall of the gastrointestinal tract [8]. Immunohistochemistry allows clinicians to differentiate leiomyosarcoma from GISTs. Leiomyosarcoma usually lacks staining for CD117, CD34 and kit [9] which are positive in the majority of GISTs [10]. By contrast, leiomyosarcoma is usually positive for smooth muscle cell markers such as actin, desmin, and h-caldesmon [9].
Most cases reported in the “pre-KIT era” as leiomyosarcoma of the stomach might be GISTs [2]. Only 19 cases of leiomyosarcoma of the stomach have been reported since early 2000s (Table 1), including our patient in this report. Patients included 11 males and 8 females. Their age ranged between 16 and 74 years with median of 51. Leiomyosarcomas of the stomach are likely to present as polypoid lesion rather than submucosal tumor or ulcerated lesion. In our case, the lesion exhibited polypoid appearance and showed progressive growth. In contrast, gastric leiomyomas and GISTs usually show submucosal tumor appearance [24]. Thus, the difference in endoscopic appearances may be the clue to discriminate leiomyosarcoma from leiomyomas and GISTs.
We happened to observe the lesion for 3 years after initial resection by ESD, because we initially thought it was a small benign polyp and our first and second biopsies failed to establish accurate diagnosis to the lesion.
Diagnosis of leiomyosarcomas relies on histopathological examination of biopsies from deeper layer because these arise between the muscularis propria and muscularis mucosa layers [2]. Conventional endoscopy usually yields superficial and normal mucosa [12]. Simple endoscopic biopsy specimens may be insufficient to establish the diagnosis due to the overlying mucosa [25]. Sampling error maybe the reason why we initially misdiagnosed the polypoid lesion as a benign polyp. Fine-needle aspiration obtained with endoscopic ultrasound guidance may be a more accurate alternative method to establish the pathological diagnosis of a submucosal tumor. We should have considered this method for this patient in an earlier stage.
Sato et al. reported the first case of primary leiomyosarcoma of the stomach that was successfully resected by ESD as a diagnostic method [1]. Pauser et al. also reported endoscopic resection of leiomyosarcoma of the stomach that was unexpectedly diagnosed because they initially suspected the lesion to be a hyperplastic polyp or an adenoma [11]. However, ESD for mesenchymal tumors in gastrointestinal tracts might lead to incomplete resection since they could originate from deeper layer of the intestinal wall [2].
The standard treatment for leiomyosarcoma is complete surgical resection of the tumor because it has malignant potential and does not respond to targeted treatment with tyrosine kinase inhibitor [1]. Previous reports have suggested that lymph node dissection is not recommended for gastric mesenchymal tumors including GISTs since most tumors rarely metastasize to regional lymph nodes [26]. However, tumors should be resected without the capsule damage because the tumor rupture is classified to high-risk group for the recurrence [27]. LECS is a useful approach that allows endoscopic evaluation of an intra-luminal mass such as gastric submucosal tumors and is less invasive than laparotomy [28]. We successfully completed LECS to treat leiomyosarcoma of the stomach. To the best of our knowledge, this is the first report of leiomyosarcoma of the stomach treated by this method. LECS is a procedure combining laparoscopic gastric resection with ESD for local resection of gastric tumors with appropriate minimal surgical resection margins [29]. Most of gastric mesenchymal tumors are recognized intra-luminally on the mucosal side and these tumors sometimes cannot be visualized laparoscopically from the outside of the stomach. It could be difficult to determine accurate resection lines for gastric submucosal tumors using conventional laparoscopic partial resection. The inappropriate resection lines may result in the positive resection margins. Therefore, endoscopic determination of an accurate cutting line is critical for the complete resection of tumors in the stomach [29]. In our case, the tumor margin was marked by injecting indigo carmine and hyaluronic acid into the submucosal layer. The stomach wall was then dissected along the marked incision line laparoscopically and we could obtain the appropriate margin.
Recently, non-exposed endoscopic wall-inversion surgery (NEWS) or combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure techniques (CLEAN-NET) were developed as a full-thickness resection without intestinal perforation [29]. NEWS or CLEAN-NET are alternative methods to resect tumor while preventing tumor cell from seeding into the abdominal cavity. Based on the pathological findings of biopsy specimen, the lesion was thought to be mesenchymal lesion, suggesting it was covered with normal mucosa.
So, we adapted LECS to the lesion and the lesion was histopathologically covered with normal mucosa (Fig. 3b).
Our report indicates that mesenchymal tumors of the stomach including leiomyosarcoma can be difficult to establish the diagnosis from conventional endoscopic biopsy specimens. LECS can be useful to establish the pathological diagnosis and simultaneously allow a curative resection of the gastric submucosal tumor.
Conclusion
Leiomyosarcoma of the stomach is extremely rare. This is the first report of leiomyosarcoma of the stomach treated by LECS. We could also follow its appearance change through endoscopic examination for 3 years.
Availability of data and materials
Not applicable.
Abbreviations
- ESD:
-
Endoscopic submucosal dissection
- GIST:
-
Gastrointestinal stromal tumor
- LECS:
-
Laparoscopic and endoscopic cooperative surgery
- EGD:
-
Esophagogastroduodenoscopy
- NEWS:
-
Non-exposed endoscopic wall-inversion surgery
- CLEAN-NET:
-
Combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure techniques
References
Sato T, Akahoshi K, Tomoeda N, et al. Leiomyosarcoma of the stomach treated by endoscopic submucosal dissection. Clin J Gastroenterol. 2018;11(4):291–6. https://doi.org/10.1007/s12328-018-0838-4.
Kang WZ, Xue LY, Tian YT. Leiomyosarcoma of the stomach: a case report. World J Clin Cases. 2019;7(21):3575–82. https://doi.org/10.12998/wjcc.v7.i21.3575.
Sahli N, Khmou M, Khalil J, et al. Unusual evolution of leiomyosarcoma of the rectum: a case report and review of the literature. J Med Case Reports. 2016;10(1):249. https://doi.org/10.1186/s13256-016-1047-8.
Yamamoto H, Handa M, Tobo T, et al. Clinicopathological features of primary leiomyosarcoma of the gastrointestinal tract following recognition of gastrointestinal stromal tumours. Histopathology. 2013;63(2):194–207. https://doi.org/10.1111/his.12159.
Agaimy A, Wünsch PH. True smooth muscle neoplasms of the gastrointestinal tract: morphological spectrum and classification in a series of 85 cases from a single institute. Langenbecks Arch Surg. 2007;392(1):75–81. https://doi.org/10.1007/s00423-006-0092-y.
Marko J, Wolfman DJ. Retroperitoneal leiomyosarcoma from the radiologic pathology archives. Radiographics Rev Publ Radiol Soc North Am, Inc. 2018;38(5):1403–20. https://doi.org/10.1148/rg.2018180006.
Min YW, Park HN, Min BH, et al. Preoperative predictive factors for gastrointestinal stromal tumors: analysis of 375 surgically resected gastric subepithelial tumors. J Gastrointest Surg: Off J Soc Surg Alimentary Tract. 2015;19(4):631–8. https://doi.org/10.1007/s11605-014-2708-9.
Beham A, Schaefer IM, Cameron S, et al. Duodenal GIST: a single center experience. Int J Colorectal Dis. 2013;28(4):581–90. https://doi.org/10.1007/s00384-012-1432-8.
Hilal L, Barada K, Mukherji D, et al. Gastrointestinal (GI) leiomyosarcoma (LMS) case series and review on diagnosis, management, and prognosis. Med Oncol (Northwood, London, England). 2016;33(2):20. https://doi.org/10.1007/s12032-016-0730-3.
Miettinen M, Lasota J. Histopathology of gastrointestinal stromal tumor. J Surg Oncol. 2011;104(8):865–73. https://doi.org/10.1002/jso.21945.
Pauser U, Grimm H. Intramucosal leiomyosarcoma of the stomach following hereditary retinoblastoma in childhood—a case report and review of the literature. World J Surg Oncol. 2008;6:131. https://doi.org/10.1186/1477-7819-6-131.
Hasnaoui A, Jouini R, Haddad D, Zaafouri H, Bouhafa A, Ben Maamer A, et al. Gastric leiomyosarcoma and diagnostic pitfalls: a case report. BMC Surg. 2018;18(1):62. https://doi.org/10.1186/s12893-018-0393-4.
Mehta V, Rajawat M, Rastogi S, et al. Leiomyosarcoma of the stomach with metastasis to the liver: a case report with review of the literature. Future Sci OA. 2018;4(2):FSO264. https://doi.org/10.4155/fsoa-2017-0100.
Insabato L, Masone S, Campione S, et al. Coexistence of primary gastric giant cell-rich leiomyosarcoma and gastrointestinal stromal tumor: report of a very rare combination and review of the literature. Int J Surg Pathol. 2012;20(1):74–8. https://doi.org/10.1177/1066896911414018.
Soufi M, Errougani A, Chekkof RM. Primary gastric leiomyosarcoma in young revealed by a massive hematemesis. J Gastrointest Cancer. 2009;40(1–2):69–72. https://doi.org/10.1007/s12029-009-9080-0.
Geraci G, Pisello F, Sciumè C, et al. Unusual acute onset of pedunculated extragastric leiomyosarcoma. Case report G Chir. 2007;28(6–7):265–9.
Damiano G, Di Ganci S, Palumbo VD, et al. Gastric leiomyosarcoma: case report and review of literature. Clin Ter. 2012;163(4):e181–4.
Masuzawa N, Kishimoto M, Nishimura A, et al. Gastric leiomyosarcoma manifesting peculiar findings: radiological-pathological correlation. Pathol Int. 2009;59(5):306–11. https://doi.org/10.1111/j.1440-1827.2009.02370.x.
Insabato L, Di Vizio D, Ciancia G, et al. Malignant gastrointestinal leiomyosarcoma and gastrointestinal stromal tumor with prominent osteoclast-like giant cells. Arch Pathol Lab Med. 2004;128(4):440–3. https://doi.org/10.1043/1543-2165(2004)128%3c440:MGLAGS%3e2.0.CO;2.
Aggarwal G, Sharma S, Zheng M, et al. Primary leiomyosarcomas of the gastrointestinal tract in the post-gastrointestinal stromal tumor era. Ann Diagn Pathol. 2012;16(6):532–40. https://doi.org/10.1016/j.anndiagpath.2012.07.005.
Rastogi S, Kalra K, Manasa P, et al. Long lasting response of trabectedin in patient with gastric leiomyosarcoma with liver metastasis: an update to previous report. Future Sci OA. 2019;6(1):FSO432. https://doi.org/10.2144/fsoa-2019-0085.
Cheng CS, Chen L, Xie J, et al. Multimodality palliative treatment with transarterial chemoembolization and high-intensity focused ultrasound for gastric leiomyosarcoma multiple liver metastasis pain: a case report. Medicine (Baltimore). 2019;98(39):e17328. https://doi.org/10.1097/MD.0000000000017328.
Rou WS, Ju JS, Kang SH, et al. A case of gastric leiomyosarcoma with multiple metastases. Korean J. 2015;65(2):112–7. https://doi.org/10.4166/kjg.2015.65.2.112.
Saito S, Yan C, Fukuda H, et al. Synchronous gastric leiomyoma and intramuscular abdominal wall granular cell tumor with similar imaging features: a case report. Int J Surg Case Rep. 2018;44:207–11. https://doi.org/10.1016/j.ijscr.2018.03.001.
Niimi K, Goto O, Kawakubo K, et al. Endoscopic ultrasound-guided fine-needle aspiration skill acquisition of gastrointestinal submucosal tumor by trainee endoscopists: a pilot study. Endoscopic ultrasound. 2016;5(3):157–64. https://doi.org/10.4103/2303-9027.183970.
Matsuda T, Nunobe S, Kosuga T, et al. Laparoscopic and luminal endoscopic cooperative surgery can be a standard treatment for submucosal tumors of the stomach: a retrospective multicenter study. Endoscopy. 2017;49(5):476–83. https://doi.org/10.1055/s-0043-104526 (Epub 2017 Apr 10).
Akahoshi K, Oya M, Koga T, et al. Current clinical management of gastrointestinal stromal tumor. World J Gastroenterol. 2018;24(26):2806–17. https://doi.org/10.3748/wjg.v24.i26.2806.
Aoba T, Kato T, Hiramatsu K, et al. A case of gastric glomus tumor resection using laparoscopy endoscopy cooperative surgery (LECS). Int J Surg Case Rep. 2018;42:204–7. https://doi.org/10.1016/j.ijscr.2017.12.023.
Hiki N, Nunobe S. Laparoscopic endoscopic cooperative surgery (LECS) for the gastrointestinal tract: updated indications. Ann Gastroenterol Surg. 2019;3(3):239–46. https://doi.org/10.1002/ags3.12238.
Acknowledgements
The authors would like to thank all the individuals who contributed to this work and for helpful discussions.
Funding
All authors have no funding regarding this paper.
Author information
Authors and Affiliations
Contributions
TT, SS, YK, KT, RK, KK, YH, JK, HO, NS were engaged in the patient’s care including the surgery and prepared the manuscript. SS and SY helped in drafting the manuscript and interpretation. HK contributed the pathological diagnosis. All authors have read and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Informed consent for the publication of this case report was obtained from the patient.
Competing interests
All authors declare no conflicts of interest regarding the publication of this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takagi, T., Saito, S., Yokota, S. et al. Laparoscopic and endoscopic cooperative surgery for leiomyosarcoma of the stomach: a case report with a review of the literature. surg case rep 7, 146 (2021). https://doi.org/10.1186/s40792-021-01218-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40792-021-01218-3