Abstract
Continuous renal replacement therapy (CRRT) is widely used for treating critically-ill patients in the emergency department in China. Anticoagulant therapy is needed to prevent clotting in the extracorporeal circulation during CRRT. Regional citrate anticoagulation (RCA) has been shown to potentially be safer and more effective and is now recommended as the preferred anticoagulant method for CRRT. However, there is still a lack of unified standards for RCA management in the world, and there are many problems in using this method in clinical practice. The Emergency Medical Doctor Branch of the Chinese Medical Doctor Association (CMDA) organized a panel of domestic emergency medicine experts and international experts of CRRT to discuss RCA-related issues, including the advantages and disadvantages of RCA in CRRT anticoagulation, the principle of RCA, parameter settings for RCA, monitoring of RCA (mainly metabolic acid–base disorders), and special issues during RCA. Based on the latest available research evidence as well as the paneled experts’ clinical experience, considering the generalizability, suitability, and potential resource utilization, while also balancing clinical advantages and disadvantages, a total of 16 guideline recommendations were formed from the experts’ consensus.
Similar content being viewed by others
Background
Due to limited resources of critical therapy could not fully meet growing medical requirements in China, some continuous renal replacement therapy (CRRT) treatments for emergent and critically-ill patients had to be performed in emergency departments. For the success of these CRRT treatments, anticoagulant therapy was essential, which was needed to prevent clotting in the extracorporeal circulation during CRRT. Previously systemic heparin anticoagulation (SHA) had been the most widely used anticoagulation method for CRRT. However, SHA had many contraindications in emergent and critically-ill patients and also had a high complication rate limiting its application, e.g., hemorrhage or heparin induced thrombocytopenia (HIT). Therefore, some new anticoagulants and methods had been explored and developed, which included regional citrate anticoagulation (RCA). After nearly a decade of clinical practice, RCA had been shown to be safer and more effective than SHA and was now recommended as the preferred anticoagulant method for CRRT [1]. RCA was increasingly used in CRRT by emergency physicians in China. However, treatments failure and complications often occurred due to the lack of unified management guidelines in the world and inappropriate therapy in clinical practice, leading to the difficulty of wide application and popularization for RCA therapy. Therefore, it is necessary to develop standardized management guidelines for RCA therapy in China and around the world. In order to provide the latest clinical practice guidance, the Emergency Medical Doctor Branch of the Chinese Medical Doctor Association (CMDA) organized a panel of domestic emergency medicine experts and international experts to discuss RCA-related issues, and formed guideline recommendations from the experts’ consensus based on the latest available research evidence as well as the paneled experts’ clinical experience.
Methods
This guideline was initiated and developed by the Emergency Medical Doctor Branch of the CMDA and it had been registered on the Practice Guideline Registration for Transparency (PREPARE, http://www.guidelines-registry.org) with the registration number of PREPARE-2021CN332. The guideline development group was composed of consensus expert group, in which two of them as secretary group and three consultant experts outside China, with 45 members. Experts with abundant experience in the application of RCA were selected, which were representative of regions and disciplines, covering emergency medicine, critical care medicine and guideline methodology, etc. Their main responsibilities were to screen relevant documents and gradually refine guideline recommendations. The secretary group was responsible for the organization, coordination and management of experts seminar, as well as proofreading of the guideline recommendations.
This guideline focused on the clinical management of RCA during CRRT. The English literature related to clinical management of RCA retrieval was based on a broad search of the PubMed, Medline and Cochrane databases, mainly from January 1960 to March 2023. The search terms were “continuous renal replacement therapy”, “CRRT”, “anticoagulation”, “regional citrate anticoagulation”, “RCA”, “citrate” and “Emergency Department”, combined with “AND” and “OR”. Chinese literature retrieval was performed on the CNKI, Wanfang and VIP databases.
The members of the expert group were divided into different fields, and according to the predetermined scope, the recommendations related to key clinical question, evidence and interpretation were initially drawn up. The core members of the expert group integrated the documents and wrote the full text of the guideline. The secretary group summarized the recommendations by organizing 22 discussions and revisions with the expert group members through online meetings. For each recommendation, the Delphi survey was conducted among all members of the consensus expert group by questionnaire survey. The total number of experts surveyed was 42. The design and content of the questionnaire were completed by the members of the secretary group, and were reviewed and published by the members of the expert group. The questionnaire mainly included the Likert scale score for each recommendation and the comments and suggestions area that could be filled in freely. For each recommendation, experts used the Likert scale to score, with a full score of 7 points, 7 points for very agree, 6 points for agree, 5 points for general agree, 4 points for uncertain, 3 points for not very agree, 2 points for disagree, and 1 point for completely disagree. The consensus setting: for a single recommendation, if more than 75% of experts with a score of ≥ 6 points, the experts reached a consensus for this recommendation. A total of 16 proposed recommendations had been reached consensus. Consensus strength for each recommendation was marked with “agreement degree”. Agreement degree = experts with score ≥ 6/total number of experts participating in the evaluation × 100%.
Finally, corresponding tools were used to evaluate the quality of the study and determine the documentary evidence. Based on the quality of evidence, the Grading of Recommendations Assessment, Development and Evaluation (GRADE) guidelines [2], and considering the generalizability, suitability, and potential resource utilization, while also balancing clinical advantages and disadvantages, levels of evidence and strengths of recommendation were established (Table 1).
Recommendation statements for management of RCA during CRRT
Advantages of RCA in CRRT anticoagulation therapy
Recommendation 1
RCA is recommended as the preferred anticoagulation method for emergent and critically-ill patients receiving CRRT if without contraindication. (Agreement degree: 92.9%, Evidence quality I, Recommendation strength A).
Remarks
SHA was once the standard anticoagulation for CRRT, which helped ensure that blood in extracorporeal circulation did not coagulate. Unfortunately, SHA has many disadvantages, such as contraindications in patients with active bleeding or those with a high risk of bleeding, weight-based (individualized) dosing, many interactions affecting anticoagulant monitoring, a high incidence of bleeding complications, and HIT.
RCA has a longer extracorporeal circulation circuit lifespan than SHA. The duration of the circuit (especially the filter) is associated with many variables, such as a patient’s coagulation status, the location of their vascular access, the CRRT mode, and parameter settings. Studies comparing the effects of different anticoagulation methods on the lifespan of the circuit have significant heterogeneity. A Meta-analysis of 30 studies comparing the effects of RCA and SHA on CRRT circuit lifespan showed that filter coagulation was significantly delayed in the RCA group compared with the SHA group (mean difference 15.69 h, 95%CI 9.30–22.08). In addition, a subgroup analysis of different modes also showed that the RCA group had a longer circuit lifespan [3]. In another prospective multicenter randomized controlled study, RCA significantly reduced system downtime in the first 72 h of treatment (1 h vs. 3 h), had fewer treatment interruptions due to coagulation (24% vs. 51%), or filter replacements (9% vs. 30%) when compared with SHA [4]. A longer filter lifespan and fewer treatment interruptions mean more efficient CRRT delivery [5].
Compared with SHA, RCA has a lower incidence of bleeding and less need for blood transfusions [6]. Two meta-analyses compared the incidence of bleeding during CRRT between SHA and RCA, and showed a lower risk of bleeding in the RCA group (RR = 0.31, 95%CI 0.19–0.51; RR = 0.34, 95%CI 0.17–0.65, respectively) [3, 7]. In a study of critically-ill patients with acute kidney injury after cardiac surgery, RCA not only prolonged the filter lifespan, but also significantly reduced the need for transfusions compared with SHA (0.29 vs. 0.62 blood units/d, P = 0.017) [8]. Since blood in the CRRT extracorporeal circulation circuit (about 150 to 200 ml of blood) will often fail to return if there is clotting in the extracorporeal circuit or filter, it makes sense that fewer blood transfusions are needed with RCA compared with SHA [9].
Other advantages of RCA include [4, 10,11,12]: 1) No side effects such as leukopenia and thrombocytopenia associated with using heparin; 2) Citrate is a physiological substance in the body with biocompatibility advantages; 3) Some studies have shown that low serum calcium during RCA use may be beneficial to inflammatory responses; 4) Due to the lower filter replacement ratio, there may be a better total cost/benefit for using RCA.
Nevertheless, strong evidence about the impact of RCA vs. SHA on survival is still lacking. Two multicenter randomized studies enrolled 170 and 212 CRRT patients, respectively, to compare the impact on survival between using SHA or RCA and found no statistical difference in survival between the two groups [13, 14]. Furthermore, two meta-analyses compared mortality between the RCA and SHA groups, and also found no advantage for RCA [3, 15]. The ongoing RICH study is the largest prospective multicenter randomized clinical trial to date and aims to add further evidence to address the relationship between CRRT anticoagulant regiments and patient outcomes [16].
Citrate is contraindicated in patients with liver disease or shock states in Kidney Disease Improving Global Outcomes (KDIGO) guidelines [1]. Recent studies suggested that liver disease and shock states were not absolute contraindications for RCA, and these would be discussed in detail below. Regardless, RCA use was at high risk for patients with liver disease and shock, it should be treated with caution.
Principles of RCA
Recommendation 2
It is recommended to set initial citrate dose based on blood flow rate, and the citrate dose should be appropriately adjusted according to the monitoring value of filter ionized calcium (iCa). (Agreement degree: 97.6%, Evidence quality I, Recommendation strength A).
Remarks
Citrate is widely found in various tissues and fluids in the human body. It is not only an important component of the skeletal system but also an intermediate product of the metabolism of glucose, lipids, and some amino acids. It plays an important role in energy metabolism. In normal circumstances, the serum concentration of citrate is extremely low, about 0.1 mmol/L, mainly in a form of stable soluble calcium citrate.
Citrate contains three carboxyl groups that can dissociate into the trivalent anion of Citrate3−. Because of the characteristics of its structure, two negatively charged carboxyl groups in Citrate3− can chelate with divalent cations such as iCa or magnesium in blood and form monovalent anions like calcium citrate or magnesium citrate (Fig. 1). The chelation reaction is rapid and the chelate does not dissociate spontaneously in the circulation. The space distance between the two positive charges of iCa matches better with the distance between the two negative charges of the Citrate3− carboxyl groups, resulting in stronger citrate-calcium complexes (CCC).
When citrate is infused into the blood, it rapidly chelates the iCa in the blood to form CCC (equivalent to changing the iCa to binding calcium), thus reducing the level of iCa in the blood. iCa also called coagulation factor IV which is vital for multiple steps of coagulation process and platelet interactions. In the endogenous coagulation pathway, iCa can assist in activating factor XI, and activate factor X together with factor VIII and activated factor IX. In the exogenous coagulation pathway, factor X is activated by iCa, factors III and VII. In the common pathway, fibrinogen can be converted into fibrin monomers by assistance of iCa, factor V and activated factor X. In addition, it can also assist in activating factor XIII and continue to assist factor XIII in transforming soluble fibrin monomers into stable fibrin polymers. The exogenous and endogenous coagulation pathway steps would be blocked if the blood level of iCa decreases obviously. Under physiological conditions, the concentration of serum iCa is 1.0–1.2 mmol/L. The blood coagulation will not occur if the blood level of iCa is reduced to below 0.35–0.4 mmol/L [17, 18].
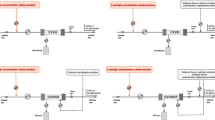
During CRRT, when citrate is infused at the access end of the extracorporeal circulation, the concentration of calcium in the extracorporeal circulation is rapidly reduced to below 0.4 mmol/L, thus preventing clotting. The negative univalent CCC formed by the chelation reaction is a small molecule (298 Dalton) with good water solubility and a sieving coefficient of about 1, which can be rapidly removed by the filter through its semi-permeable membrane [19, 20]. Due to different CRRT modes and therapeutic doses, about 30–60% of CCC molecules will enter the effluent through the permeable membrane. The CCC molecules which are not removed via the hemofilter enter the systemic circulation and are first metabolized into citric acid rapidly in body cells. Citric acid is metabolized through the tricarboxylic acid cycle, which is oxygen dependent and mainly occurs in organs with high mitochondrial content. In physiological conditions, it is mainly metabolized in liver and a small portion is metabolized in skeletal muscles. The half-life of CCC under physiological conditions is only 5 min. CCC is eventually decomposed into bicarbonate through the tricarboxylic acid oxidation cycle (one molecule of citrate yields three molecules bicarbonate), and the iCa is released back into the blood. Meanwhile, the unchelated calcium ions are also partially removed from the circuit blood into the effluent (Fig. 2). As a result, severe hypocalcemia can occur if ongoing supplementation of iCa is not provided. Therefore, adequate amounts of iCa must be supplied directly to the patient intravenously or through the return end of extracorporeal circulation circuit to maintain physiological iCa level. RCA anticoagulation is thus achieved only in the CRRT circuit (i.e., regional anticoagulation) with little interference with in vivo coagulation processes (Fig. 3).
Schematic diagram of citrate metabolism. When trisodium citrate is infused into the extracorporeal circulation, citrate-calcium complexes (CCC) is formed by the chelation reaction of ionized calcium (iCa) and Citrate3−. iCa is rapidly reduced to prevent clotting. About 30–60% of CCC molecules enter the effluent through the permeable membrane depending on different continuous renal replacement therapy (CRRT) modes and therapeutic doses. The residual CCC molecules return to the systemic circulation and are metabolized rapidly in body cells. The half-life of CCC under physiological conditions is only 5 min. CCC is eventually decomposed into bicarbonate through the tricarboxylic acid oxidation cycle (one molecule of citrate yields three molecules bicarbonate), and the iCa is released back into the blood
Schematic diagram of RCA in the CRRT system. Citrate is infused into the extracorporeal circulation before filter. Citrate-calcium complexes (CCC) is formed by the chelation reaction of ionized calcium (iCa) and Citrate3−. Partial CCC and iCa are removed by the filter. As a result, iCa in the filter is rapidly reduced to below 0.4 mmol/L, thus preventing clotting. The concentration of iCa in the filter is routinely monitored at the site behind the filter. Before the blood is returned to the body, additional iCa is infused to the blood in order to replenish the calcium removed to effluent by the filter. RCA regional citrate anticoagulation, CRRT continuous renal replacement therapy
When RCA is used, citrate is added at the access end of extracorporeal circuit to ensure a citrate concentration of 3–4 mmol/L (i.e., 3–4 mmol citrate per 1 L of whole blood) [9, 21,22,23,24,25]. The flow rate of citrate is then adjusted by monitoring the concentration of iCa in the filter (hereinafter referred to as iCa in the filter, with the sampling site residing behind the filter, as shown in Fig. 3), so as to ensure the effectiveness of anticoagulation with the concentration of iCa in the filter ranging from 0.2 to 0.4 mmol/L. In order to supplement the calcium lost in the effluent through the filter membrane, extra iCa is infused in the return end of the extracorporeal circuit or directly to patients via central vein to ensure normal serum calcium in the patient. It is very important to monitor the concentration of iCa in patients’ systemic circulation (hereinafter referred to as iCa in the body, sampled from the patient’s artery or vein).
Parameter settings for RCA
Setting the initial flow rate for citrate and scheme adjustment
Recommendation 3
It is preferable to use a calcium-free solution as a replacement fluid in predilution continuous veno-venous hemofiltration (CVVH) or as a dialysate in continuous veno-venous hemodialysis (CVVHD). If a replacement fluid containing calcium is used and predilution CVVH or CVVHD mode is selected during RCA, it is recommended to adjust the citrate dose appropriately to achieve targeted filter iCa concentration. (Agreement degree: 97.6%, Evidence quality III, Recommendation strength B).
Recommendation 4
The recommended target value of iCa in the filter is 0.2–0.4 mmol/L, but the target value should be individually adjusted according to the treatment situation. (Agreement degree: 100.0%, Evidence quality II, Recommendation strength C).
Remarks
Currently, there are two forms of Citrate3− solutions available for anticoagulation: one is only used as an anticoagulant (known as citrate anticoagulant). It includes 4% trisodium citrate (TSC) solution and acid-citrate-dextrose formula A (ACD-A). ACD-A is a type of fluid used for blood preservation but is now less commonly used in CRRT for anticoagulation. The other form of solution is a kind of buffer solution containing Citrate3− (balanced solution) which can be used for anticoagulation and as a predilution replacement fluid, known as citrate replacement solution. Currently, there are mainly manual citrate-based replacement solution (balanced solution) and commercial solutions (e.g., prismocitrate 10/2, prismocitrate 18/0). Citrate-based replacement solution cannot be used for postdilution or dialysis. Citrate3− contents, electrolyte concentration and physicochemical properties of the above solutions are listed in Table 2 [26].
There is a corresponding relationship between citrate flow rate and blood flow rate. The initial flow rate of citrate should be determined based on the blood flow rate to reach a citrate concentration of about 3–4 mmol/L in the extracorporeal circulation, as shown in Table 3. Considering the adequacy of initial anticoagulation and the practical experience of each expert, the guideline suggests that if calcium free replacement fluid is used, the initial flow rate of citrate solution should be: 1) ACD-A (ml/h) = blood flow rate (ml/min) × 1.8; 2) 4% TSC solution (ml/h) = blood flow rate (ml/min) × 1.5. If replacement fluid containing calcium is used and predilution CVVH or CVVHD mode is chosen (iCa concentration in commercial replacement fluid is usually 1.6 mmol/L in China, see Additional file 1: Table S1), the flow rate of citrate solution should be increased to chelate iCa in the replacement fluid. The initial flow rate is empirically suggested to be as follows: 1) ACD-A (ml/h) = blood flow rate (ml/min) × 2.0; 2) 4% TSC solution (ml/h) = blood flow rate (ml/min) × 1.7.
Filter calcium levels should be closely monitored during the first 24 h of treatment (usually measured with a blood gas analyzer). It is recommended to conduct the first detection 30 min after the start of treatment, and then monitor iCa every 2 h for 4 consecutive times. Adjust the flow of citrate according to the level of iCa in the filter, and then monitor it once every 4 h for 4 consecutive times after it is deemed stable. If the treatment goes well, monitoring can be changed to once every 6 h after 24 h, i.e., (30 min) → (q2 h × 4) → (q4 h × 4) → (q 6 h × 4). According to the measured concentration of iCa in the filter, the citrate flow rate can be adjusted by referring to Table 4.
It should be emphasized that adequate anticoagulation in the early CRRT phase is very important because the cascade of amplification reactions may accelerate clotting once microthrombi are formed in the filter. Therefore, iCa should be closely monitored after the start of treatment, and the program should be actively adjusted to achieve stabilization of anticoagulation as soon as possible.
From the perspective of the principles of using RCA, factors determining the dose of citrate include: 1) Blood flow rate. The citrate flow rate must match the blood flow rate to ensure a stable anticoagulant concentration in the extracorporeal circulation and should be adjusted accordingly after a change in the blood flow rate. 2) The site where replacement fluid is added. If a calcium-free replacement fluid is used in predilution mode, the blood entering the filter will be diluted, so that the iCa concentration in the filter will decrease, and the demand for citrate will decrease accordingly. 3) Composition of replacement fluid. Whether the replacement fluid contains calcium and magnesium also affects the flow rate of citrate. If calcium-containing replacement fluid is used in predilution mode, the amount of iCa entering the filter will increase, requiring more citrate to chelate them. If calcium-containing replacement fluid is used in postdilution mode, the impact on the amount of citrate will be small, but may increase the risk of coagulation in the post-filter air trap chamber. The blood sampling location of the iCa in the filter is usually before the infusion site of the postdilution replacement fluid, and the post-filter air trap chamber is usually located behind the input point of the postdilution replacement fluid, therefore the actual iCa concentration in the post-filter air trap chamber is higher than the measured value. 4) Individual anticoagulation intensity target. The anticoagulant intensity required in vitro may vary depending on the coagulation state of the patient. There is a huge individual difference in the iCa threshold to prevent blood coagulation, such as satisfactory anticoagulation is achieved in some patients when iCa in the filter are only less than 0.5 mmol/L, while in some others, they are required to be 0.2 mmol/L strictly [27,28,29,30,31]. In addition, error measurement of iCa in post-filter also prompts that anticoagulation intensity should not be set only based on the level of iCa. There are ongoing concerns of technologies when measuring "post-filter" iCa. These technologies have been developed to measure ionized Ca in the physiologic range, however there is great uncertainty in the accuracy of measurement of iCa when measuring in subphysiologic range using blood gas analyzers. Therefore although the target range of 0.2–0.4 mmol/L of iCa in the filter is appropriate for most patients, in practice the target value should be individualized according to the actual treatment process rather than being restricted to this range [32]. Especially when clotting start to occur in the filter, it would call for empirical increase of citrate infusion rate to minimize clotting propensity despite the level of iCa is in the range in post-filter.
Calcium supplementation and scheme adjustments
Recommendation 5
Calcium supplementation is recommended to maintain in vivo physiological levels during RCA (Agreement degree: 100.0%, Evidence quality II, Recommendation strength A). Calcium supplementation can be administered through the return end of the extracorporeal circulation circuit or directly through the central vein, rather than directly through a peripheral vein (Agreement degree: 100.0%, Evidence quality III, Recommendation strength B).
Recommendation 6
If calcium-free replacement fluid is used during RCA, it is recommended that the initial flow of 5% calcium chloride infusion be set to an effluent flow rate divided by 200 or 10% calcium gluconate infusion be set to an effluent flow rate divided by 125; If a calcium-containing replacement fluid is used and predilution CVVH or CVVHD mode is selected, an initial flow of 5 ml/h for 5% calcium chloride infusion or 8 ml/h for 10% calcium gluconate infusion is recommended. (Agreement degree: 100.0%, Evidence quality III, Recommendation strength C).
Recommendation 7
During RCA, it is recommended to adjust the calcium supplementation rate according to the monitoring in vivo value of iCa (Agreement degree: 100.0%, Evidence quality II, Recommendation strength A). The recommended target in vivo value of iCa is 0.9–1.1 mmol/L (Agreement degree: 90.4%, Evidence quality II, Recommendation strength C).
Remarks
In order to ensure patient safety, it is necessary to closely monitor the in vivo iCa level during RCA to prevent serious abnormal blood calcium levels. In vivo iCa monitoring should be obtained from the patient’s body (either arterial or venous sampling) or from the access end of the extracorporeal circulation circuit (prior to the site of citrate addition). Some machines provide a special blood sampling site at the access end of the extracorporeal circulation circuit.
The physiological level of in vivo iCa is 1.0–1.2 mmol/L. With the exception of some situations (e.g., hyperkalemia), hypocalcium-related symptoms usually do not occur with iCa concentration above 0.8 mmol/L. If the optimal target value of iCa is set slightly below a typical physiological level, such as 0.9–1.0 mmol/L, the amount of citrate required may be reduced. In addition, a slightly lower calcium target may help to reduce systemic inflammation [18, 33,34,35,36].
In China, 5% calcium chloride and 10% calcium gluconate are commonly used for iCa supplementation, and the difference in iCa concentration between them is about 1.5 times (iCa concentration in 5% calcium chloride solution is about 1.5 times that of 10% calcium gluconate, see Table 5). Additional iCa supplements can be administered at the return end of the extracorporeal circulation circuit or directly through a central venous catheter. Because calcium chloride is a strong vascular irritant and extravascular leakage that may occur during infusions can cause tissue necrosis, peripheral venous infusion should be avoided. 10% calcium gluconate infusion also carries a similar risk and is not recommended for peripheral infusion.
As for setting the initial flow rate of calcium supplementation, there are some empirical formulas to estimate the starting flow rate of calcium supplementation according to the citrate flow rate. However, considering the metabolic process of citrate, the rate of calcium supplementation is not necessarily related to the flow rate of citrate, but is determined by the total amount of calcium lost through the effluent. Due to the complex balance between binding calcium and iCa in the circulation when partial iCa is chelated by citrate during CRRT, and the difference in sieving coefficient between calcium citrate and iCa, it is difficult to accurately calculate the dose of calcium supplement through therapeutic parameters. Studies to help determine the best initial rate of calcium supplementation are also lacking. This guideline recommends using empirical formulas for the initial settings. If a calcium-free replacement fluid is used, it is recommended that the initial flow rate of 5% calcium chloride infusion is set to an effluent flow rate divided by 200, or, if using a 10% calcium gluconate infusion, it should be set to an effluent flow rate divided by 125. If a calcium-containing replacement fluid is used and a predilution CVVH or CVVHD mode is selected, an initial flow rate of 5 ml/h if using a 5% calcium chloride infusion or 8 ml/h if using a 10% calcium gluconate infusion is recommended. This empirical initial value may not be appropriate for all patients, but if standardized iCa monitoring is performed, it should not result in severe blood calcium abnormalities during the initial few hours because of the considerable buffering capacity of the calcium pools in the body. It should be emphasized that the above empirical values are only for making initial settings, and the calcium supplement rate must be further adjusted according to the level of in vivo iCa. It is suggested to monitor the in vivo iCa level according to the previously stated plan of (30 min) → (q2 h × 4) → (q4 h × 4) → (q6 h × 4) after starting treatment, and adjust the calcium supplement rate according to the plan in Table 6.
CCC formed by chelating iCa is a soluble small molecule substance, part of which enters the effluent and means there will be a net loss of chelated calcium, while the remainder (i.e., unchelated iCa) is returned to the body. Some of the unchelated iCa in the extracorporeal circulation also pass through the filter into the effluent. Therefore, the amount of iCa that needs to be supplemented is actually the total net loss of calcium in the effluent. All the factors that affect the permeability of the semi-permeable membrane and the flow rate of effluent can affect the amount of iCa supplementation required. Accordingly, the factors influencing iCa supplementation include: 1) All factors that increase the flow rate of the replacement fluids (pre-dilution + post-dilution + dialysate) will increase calcium loss, and changes to these parameters may affect the calcium supplementation rate. 2) When using a replacement fluid containing calcium, although the ionized calcium concentration in commercial replacement fluid (e.g., 1.6 mmol/L in China, See Additional file 1: Table S1 for more details) is often higher than physiological concentration in blood, calcium supplementation may still be required, but at a reduced amount compared to the use of calcium-free replacement fluids [24, 25, 37,38,39]. 3) As the treatment time lengthens, the efficiency of the semi-permeable membrane gradually decreases, and the loss of calcium through the filter will be reduced if other parameters remain unchanged, so the amount of supplemental calcium also decreases slightly. In addition, it should be noted that if the rate of iCa supplementation needs to be continuously increased during treatment to maintain a normal serum iCa level, the possibility that serious complications should be considered. (see below).
Monitoring of RCA
Although RCA has many advantages over SHA, it is not a perfect anticoagulation method, and serious complications may occur if monitoring is not standardized. The main complications associated with RCA include acid–base balance and electrolyte disturbances. Lacking accurate understanding of the mechanisms of complications can lead to inappropriate management. The metabolic complications associated with RCA are summarized in Table 7.
Metabolic alkalosis
Recommendation 8
Metabolic alkalosis is the most common complication of RCA, and the primary management strategy is to decrease extra bicarbonate infusion, or increase the flow rate of replacement fluid or dialysate if without extra bicarbonate infusion. (Agreement degree: 92.9%, Evidence quality II, recommendation strength C).
Remarks
Metabolic alkalosis is a relatively common and an easily controlled complication of using RCA, with an overall incidence of 29–50% [40, 41]. There are two sources of bases related to RCA, one is derived from exogenous bicarbonate supplementation, and the other is metabolite of CCC entered in the systemic circulation. When CCC is completely metabolized, it will release iCa and produce HCO3− at the same time. Taking 4% TSC solution as an example, infusing 200 ml of 4% TSC solution is equivalent to infusing 137 ml of 5% NaHCO3 solution. When the total provision of bicarbonate exceeds the therapy requirement, metabolic alkalosis will occur. If without exogenous bicarbonate infusion, metabolic alkalosis still exists, this situation is called “citrate overload”, which imply excessive CCC entering the systemic circulation and it can be completely metabolized and then produce excessive HCO3−, leading to metabolic alkalosis. “Citrate overload” is a sign of excessive citrate administration, or more frequently, of low clearance in the hemofilter [42].
The risk of metabolic alkalosis varies with different anticoagulants containing citrate. According to Stewart’s principle of acid–base equilibrium [43,44,45,46], alkalization occurs when the strong ion difference (SID) in plasma increases after citrate is metabolized in the body. Each 1 mmol of citrate provided by 4% TSC metabolizes into 3 mmol of SID, while only 2 mmol of SID are produced by ACD-A. Therefore, under the same anticoagulation strength (same amount of citrate provided), 4% TSC has a stronger alkalizing effect on plasma, leading to more cases of metabolic alkalosis. In addition, the use of different replacement fluids or dialysate may also have an impact on metabolic alkalosis. When lactate replacement solution fluid (with a lactate concentration usually of 25–45 mmol/L, and one molecule of lactate in vivo produces one molecule of HCO3−) and RCA (also producing bicarbonate) are used together, metabolic alkalosis is more likely to occur because excessive alkali produced by metabolization of lactate and citrate in the body is over the therapeutical requirements during CRRT.
The common treatment strategies for metabolic alkalosis include: 1) If other alkali agents (e.g., 5% NaHCO3) are being used, consider reducing or discontinuing such infusions; 2) Increase the flow rate of replacement fluid or dialysate to increase the proportion of CCC cleared by the semi-permeable membrane, and also increase the removal of circulating bicarbonate; 3) If without extra bicarbonate infusion and the dose of replacement fluid or dialysate is enough, it can be considered to reduce the infusion of citrate under the premise of ensuring anticoagulation effect. Reduce the flow rate of blood to match the reduced flow rate of citrate if necessary. It should be noted that lowering blood flow rate can lead to an increase in the filtration fraction of postdilution mode of CVVH/CVVHDF, which may increase the risk of thrombosis in the filter; 4) If the lactate replacement fluid is used, consider changing the replacement fluid formulation; 5) The filter should also be replaced when CCC clearance drops if caused by a decrease in filter efficiency. Infusion of saline is not recommended for the treatment of RCA-related metabolic alkalosis unless the patient also has evidence of having insufficient volume.
Metabolic acidosis
Recommendation 9
The accumulation of citrate should be monitored during RCA. The following indicators are suggested as early warning signs of citrate accumulation: 1) Total calcium/iCa is > 2.25 and increasing; 2) Gradually worsening ionized hypocalcemia; 3) The amount of iCa supplementation is gradually increasing. (Agreement degree: 95.2%, Evidence quality II, recommendation strength B).
Recommendation 10
The following actions are suggested for managing citrate accumulation: 1) Optimize hemodynamics and tissue perfusion to correct hypoxia and shock; 2) Reduce the rate of citrate infusion; 3) Finally, change anticoagulant methods if the above treatments are ineffective. (Agreement degree: 95.2%, Evidence quality III, Recommendation strength C).
Recommendation 11
When metabolic acidosis related to RCA during CRRT occurs, the following treatment strategies are suggested: 1) Check for any evidence of citrate accumulation. If citrate accumulation is the cause, follow Recommendation 10; 2) Add additional base, such as NaHCO3; 3) Reduce the replacement fluid or dialysate flow rate; 4) Increase infusion rate of citrate. (Agreement degree: 90.5%, Evidence quality III, Recommendation strength B).
Remarks
Metabolic acidosis may occur during RCA, involving two different mechanisms with different management strategies.
Citrate accumulation
The body’s ability to metabolize citrate is limited, but it’s still not clear what the maximum is. With an increase in the Citrate3− infusion rate during RCA, CCC concentration in the systemic circulation also increases. Limited data shows that a systemic circulation CCC concentration of less than 2–3 mmol/L may be safe [47,48,49,50,51]. Under general anticoagulant doses, the infusion rate of Citrate3− is less than 40 mmol/h, and nearly 50% of the CCC is lost in the effluent through the filter. The concentration of CCC that finally enters the body’s circulation is generally less than 2 mmol/L, which has a large margin of safety [48,49,50,51]. However, if a patient’s tricarboxylic acid cycle is significantly inhibited, such as in the presence of severe hypoxia or hypoperfusion, citrate accumulation (that is, large amounts of CCC in the bloodstream) can occur as a result of significant metabolic impairment.
Citrate accumulation is a rare but potentially fatal complication during RCA. In fact, CCC itself is not toxic, but accumulation of CCC usually occurs in patients with significant impairment of the tricarboxylic acid cycle, often accompanied with severe disease states such as hypoxia, organ failure, hypoperfusion, and lactic acidosis. In a retrospective study including 1070 patients with RCA-CRRT, citrate accumulation occurred in 32 patients, with an overall incidence of 2.99% (32/1070), and none of these 32 patients survived [52]. Another prospective study including 100 RCA-CRRT patients at high risk of bleeding after surgery showed that the incidence of citrate accumulation was 1% [41].
At present, plasma citrate levels cannot be routinely measured in most clinical laboratories, therefore citrate accumulation can only be predicted by indirect signs. The first sign is “citrate gap” which is the lingo in contemporary practice of CRRT referring to the difference between total and iCa in the United States. The total calcium level is the total of all calcium, including the ionized, protein bound and citrated calcium forms. With progressive systemic citrate accumulation, the increased citrate combines with more ionized calcium, which lead to an increase of citrated calcium, and meanwhile a decrease occurs in CCC metabolism in the systemic circulation. When the iCa released by CCC decreases, it may result in lower level of iCa. Therefore, increased total calcium, increased “citrate gap”, progressive ionized hypocalcemia or a gradually increasing requirements for calcium supplementation are the signs of citrate accumulation. Moreover, similar to the concept of “citrate gap”, a gradually increasing total-Ca/iCa ratio is highly suggestive of progressive citrate accumulation, and a ratio ≥ 2.5 often indicates the presence of significant citrate accumulation. Studies showed that total-Ca/iCa ratio is highly correlated with plasma CCC level and might be the most reliable indicator [42, 53,54,55,56]. Furthermore, as CCC metabolism decreases, plasma bicarbonate also decreases. New or worsening metabolic acidosis may be another sign of citrate accumulation. Since almost all such patients had severe organ failure and persistent hyperlactatemia, they presented as metabolic acidosis with a high anion gap. However, neither hyperlactatemia nor high anionic gap metabolic acidosis had a consistent association with plasma CCC level, and neither could reliably predict or detect citrate accumulation [53, 54]. They can only indicate a high risk for citrate accumulation.
In summary, the recommendation suggests the following indicators as warning signs of citrate accumulation [55, 56]: 1) Total-Ca/iCa ratio > 2.25 with dynamic increases; 2) Gradually worsening ionized hypocalcemia; 3) Gradually increasing requirements for intravenous calcium supplementation. It is recommended that total-Ca level be measured at least once a day to calculate the total-Ca/iCa ratio. The occurrence of citrate accumulation does not mean that anticoagulant methods must be changed. A considerable number of patients can be successfully treated by reducing the CCC load [57]. The suggested treatments are as follows: 1) Down-regulating blood flow and thus reducing citrate infusion, 2) Actively managing the patient’s circulation to correct hypoxia and shock. If the above treatments are ineffective, other anticoagulant methods should be considered.
Insufficient base delivery
Regardless of using 4% TSC solution or ACD-A as anticoagulant, CCC has the function of alkalizing plasma when it is completely metabolized in the body, that is, it is equivalent to a net input of alkali. These alkaline loads on the one hand buffer metabolic acidosis in renal injury patients and on the other hand supplement bicarbonate lost through the filter. If the net alkali load cannot meet the total demand of these two aspects, metabolic acidosis, due to insufficient citrate delivery, occurs. This type of metabolic acidosis is due to the lack of net base with normal anion gap (if there is no other metabolic acidosis causes present), which has a completely different mechanism during citrate accumulation. The following treatments are recommended: 1) Additional base supplements (e.g., 5% NaHCO3); 2) Reduce the flow rate of replacement fluid or dialysate to reduce the loss of CCC in the effluent (note the potential for an insufficient therapeutic dose); 3) Increase flow rate of citrate infusion (possibly simultaneously increase the blood flow rate).
Therapeutic dose and metabolic complications
As mentioned above, the main mechanism of metabolic acidosis or alkali poisoning complications of RCA is related to the amount of CCC entering the patient’s body and whether it can be adequately metabolized. Metabolic alkalosis can occur if excessive CCC enters the body and is completely metabolized, while metabolic acidosis occurs when the amount of CCC entering the body cannot be fully metabolized or insufficient base delivery partially caused by decreased CCC entering the body. The factors affected remove of CCC have influence on the acid–base balance. Such as semi-permeable membrane and therapeutic dose. The more CCC that is removed by the filter’s semi-permeable membrane, the less CCC will enter the body. At a certain blood flow rate, the clearance ratio of CCC through the semi-permeable membrane mainly depends on the replacement fluid or dialysate flow rate. The higher the flow rate of the replacement fluid or dialysate, the higher the CCC clearance ratio, and the less CCC remains in the patient’s serum.
When RCA is used, alkali required vary in different therapeutic doses. To maintain acid–base balance, it is necessary to calculate the additional amount of base supplementation based on the therapeutic dose and citrate infusion rate, and adjust it dynamically based on the results of blood gas analysis. Take an example for explanation. The available commercial hemofiltration basic replacement fluid (carbonate replacement fluid A, 4000 ml) is commonly used in China combined with 5% NaHCO3 (carbonate replacement fluid B, 250 ml) in order to compensate for the loss of base (See Additional file 1: Table S1 for more details), which means that when the replacement fluid is at a flow rate of 1000 ml/h, a supplement of 5% NaHCO3 at 63 ml/h is needed to maintain acid–base balance. One molecule of Citrate3− entering the body will be metabolized into three molecules of HCO3− when RCA is used. Taking 4% TSC as an example, if the flow rate is 200 ml/h, it is equivalent to 137 ml/h of net alkali (5% NaHCO3) supplementation before passing through filter. As shown in Table 8, at low replacement fluid flow rates, the demand of alkali supplementation is also at a lower level. Consequently, the net alkali load generated by RCA will result in metabolic alkalosis even without additional alkali supplementation. The alkali supplementation requirement increases with an increasing replacement fluid flow rate, consequently changing the net alkali load from positive to negative. Metabolic acidosis (normal anion gap) would occur if additional base is insufficiently supplemented (e.g., 5% NaHCO3). Therefore, when the citrate flow rate matches the blood flow rate, acid–base balance can be maintained with approximately 10 times replacement fluid flow rate to the citrate (4% TSC) flow rate. Metabolic alkalosis will occur when the replacement fluid or dialysate flow rate is significantly lower than this range, which can be corrected by increasing the replacement fluid or dialysate flow rate. Or on the contrary there will be metabolic acidosis, which requires additional alkaline supplementation (e.g., 5% NaHCO3) to maintain acid–base balance.
Electrolyte disturbances
Recommendation 12
Serum electrolyte levels, especially iCa and magnesium levels, need to be closely monitored for timely detection and treatment of electrolyte abnormalities. (Agreement degree: 97.6%, Evidence quality III, Recommendation strength B).
Remarks
Hypocalcemia or hypomagnesemia
Citrate in the extracorporeal circulation chelates iCa to form CCC, part of which along with some of the unchelated iCa will be lost in effluent through the semi-permeable membrane. Similarly, ionized magnesium can also be lost. If the missing ions are not properly replenished, hypocalcemia or hypomagnesemia will occur. Hypocalcemia reduces myocardial contractility, induces arrhythmia, tetany and other systemic symptoms. However, these complications are relatively easy to detect and manage with standard monitoring. It is recommended to monitor the iCa concentration in the filter and the blood in the body at least every 6 h. Total-Ca and total-Ca/iCa measurements are recommended once a day for patients at low risk of citrate accumulation and every 6 h for patients at high risk. The requirements for magnesium supplementation depend on the composition of the replacement fluid or dialysate being used. Thus, it is recommended to measure serum magnesium once a day.
Hypercalcemia
By following standard RCA therapy protocols and monitoring blood calcium levels, hypercalcemia is less likely to occur. In a few cases, such as citrate accumulation, increasing the amount of calcium supplement because of decreased in vivo iCa, is likely to cause high total-Ca and low iCa levels. This condition should be managed as previously discussed citrate accumulation.
Moreover, a retrospective study showed that in patients with severe hypercalcemia who underwent CVVH, RCA more effectively decreased calcium levels and had a superior filter lifespan and no obvious adverse events compared with low molecular weight heparin [58].
Hypernatremia
The impact of the altered sodium ion concentration caused by CRRT treatment needs to be considered in advance, especially when using RCA for anticoagulation. It should be noted that the increase of sodium ion may be more obvious in patients with hyponatremia, or the higher sodium might be beneficial to control brain edema.
The sodium concentration in 4% TSC solution or ACD-A is much higher than in normal serum (Table 2). There is a risk of hypernatremia from prolonged or high-dose citrate anticoagulants [25, 59]. However, hypernatremia is uncommon when following a standardized RCA protocol. The reasons for this include: 1) Although the sodium concentration in 4% TSC is high (the molar concentration is 408 mmol/L), the increased range of blood sodium concentration is not significant in patients with normal serum sodium within the commonly used parameters. 2) A considerable portion of sodium enters the effluent through the semi-permeable membrane when the sodium concentration is low in commonly used commercial replacement fluid (for example, the sodium concentration of carbonate base replacement fluid A is 113 mmol/L). If hypernatremia happens during RCA, blood sodium concentration can be reduced by increasing the replacement fluid or dialysate flow rate to allow more sodium clearance through the semi-permeable membrane. There is generally no need to reduce the citrate flow rate or adjust sodium concentration in the replacement/dialysate fluid.
However, the above discussion of uncommon hypernatremia in using RCA is based on the relatively low sodium concentration in replacement fluid A commonly used in China, whereas there are many commercial replacement/dialysate fluids outside China with a sodium content much higher than that (e.g., 140 mmol/L). When fluids with higher sodium concentrations is used, isosmolar citrate solutions could be used instead of ACD-A or 4% TSC in case occurrence of hypernatremia.
In brief, hypernatremia is not a contraindication for using RCA, however the effect on patients caused by the change of sodium concentration should be considered before apply.
Special issues during RCA
RCA and liver dysfunction
Recommendation 13
Liver dysfunction is not recommended as a contraindication in using RCA. Patients with liver dysfunction are at increased risk for citrate accumulation and should be monitored closely during treatment. (Agreement degree: 92.9%, Evidence quality II, Recommendation strength C).
Remarks
It is generally believed that patients with liver dysfunction have an impaired ability to metabolize citrate [50]. Therefore, liver dysfunction has traditionally been considered a contraindication in using RCA [47]. However, several recent studies have shown that RCA can still be used safely in patients with liver dysfunction or even liver failure, without a significant increased risk of complications [57, 60]. In a prospective multicenter observational study (7 ICUs, 133 RCA-CRRT patients), patients were classified into three groups according to the levels of serum total bilirubin: ≤ 34.2 μmol/L for normal liver function, > 34.2 to ≤ 119.7 μmol/L for mild liver failure, and > 119.7 μmol/L for severe liver failure. The results showed no difference in the incidence of safety endpoints (severe acid–base disorders of any cause or iCa abnormalities) among the three groups, and RCA was well tolerated even in patients with severe liver failure [57]. A meta-analysis that included 10 observational studies of 1241 patients with hepatic dysfunction undergoing RCA-CRRT showed no significant difference in plasma citrate levels in patients compared to those without hepatic dysfunction [60]. Several studies have shown that RCA can also be used safely in patients with renal failure after liver transplantation or in patients being treated with extracorporeal artificial liver support [61,62,63].
There are two possible explanations for the above results. First, the liver is not the only place in the body that can metabolize citrate, but kidneys, muscles and other tissues that contain the needed enzymes also can metabolize citrate. Second, traditional liver function evaluation indexes [aspartate transaminase (AST), alanine transaminase (ALT), bilirubin, cholinesterase, Child–Pugh score, model of end-stage liver disease (MELD) score, etc.] have a poor ability to predict citrate accumulation [64]. Therefore, liver failure should not be regarded as a contraindication for RCA. These patients should be regarded as a special group, and the changes of iCa and other electrolytes should be closely monitored during treatment to identify those with citrate accumulation [25, 57, 60, 64,65,66]. It is possible to use a lower citrate flow rate combined with a lower blood flow while closely monitoring the ratio of serum total calcium to serum ionized calcium [25].
RCA and lactic acidosis
Recommendation 14
Lactic acidosis is not recommended as a contraindication to RCA. But such patients are at increased risk of citrate accumulation and should be monitored closely during treatment. (Agreement degree: 92.9%, Evidence quality II, Recommendation strength C).
Remarks
CCC that enters the body is metabolized in the mitochondria through the tricarboxylic acid cycle, which is oxygen dependent. In patients with severe hypoxia, shock or mitochondrial dysfunction, citrate tends to accumulate because of the impaired tricarboxylic acid cycle induced by decreased mitochondrial oxidative chain activity. In theory, the use of RCA in these patients could be dangerous. However, clinical practice has shown that RCA use is well-tolerated in patients with shock, especially in those whose circulation was improving [53]. Therefore, there is still a lack of criteria for evaluating the severity of metabolic impairment and RCA contraindication in these patients. Lactic acid is an important marker of severe tissue hypoperfusion, severe hypoxia or mitochondrial dysfunction. Because lactate and citrate share the same metabolic pathways in mitochondria, citrate accumulation is often combined with hyperlactatemia. They are not causally related in pathophysiology, but important markers of severe metabolic impairment and disease severity. Such patients usually have a very high mortality rate [53, 64].
Retrospective studies showed that almost 100% of patients who develop citrate accumulation have lactic acidosis [52]. Lactate might be used to evaluate citrate metabolic capacity. However, in a significant proportion of patients with elevated lactate not associated with hypoxia or hypoperfusion, the tricarboxylic acid cycle in these patients may be expected to be normal without affecting citrate metabolism. Therefore, hyperlactatemia cannot be used as a diagnostic criterion for citrate accumulation, nor a contraindication for RCA. Even if there is a severe lactate accumulation due to hypoperfusion or hypoxia, RCA can be safely administered if lactic acid declines in the first few hours of treatment [53]. The study has shown that when a specific lactate threshold (e.g., 2.39 mmol/L) was used to assess the risk of citrate accumulation in RCA-CRRT patients, the negative predictive value was significant, and the positive predictive value was low [53]. Therefore, patients with persistent hyperlactatemia due to shock or hypoxia may have a higher risk of citrate accumulation than other patients, and such patients should be considered as a special group needing closer monitoring by measuring the total-Ca/iCa ratio and pH [53]. If total-Ca/iCa ratio exceeds 2.5, an intervention to reduce the citrate load or to discontinue the infusion should be considered [26].
RCA and CRRT modes
Recommendation 15
RCA is not recommended to be used in slow continuous ultrafiltration (SCUF) mode, as CCC cannot be effectively removed. RCA can be safely used in other modes such as CVVH, CVVHD, and continuous veno-venous hemodiafiltration (CVVHDF). (Agreement degree: 95.2%, Evidence quality III, Recommendation strength C).
Remarks
The modes commonly used for CRRT include SCUF, CVVH, CVVHD, and CVVHDF. SCUF mode does not require replacement fluid, and the amount of effluent generated is much lower than in other modes. If RCA is used in the SCUF mode, CCC cannot be effectively removed by the semi-permeable membrane, and the excessive CCC entering the systemic circulation makes the patient prone to metabolic alkalosis [67]. In CVVH mode, RCA can be safely used regardless of pre- or post-dilution. The main disadvantage is that it is limited by transmembrane pressure and filtration fraction when it is necessary to increase the flow rate of replacement fluid to quickly remove CCC (such as in metabolic alkalosis or citrate accumulation). CVVHD mode eliminates solutes by diffusion without limitation of transmembrane pressure and filtration fraction, and can increase dialysate flow rate to increase the proportion of CCC clearance through the semi-permeable membrane to alleviate metabolic alkalosis or reduce citrate accumulation. RCA can also be safely used in CVVHD. The main limitation of CVVHD is that the citrate replacement fluid (balanced solutions) alone cannot be used, because the citrate replacement fluid should be infused pre filter in the extracorporeal circuit to exert anticoagulant effect and also as predilution replacement fluid, which should not be used as dialysate, as shown in Table 2. CVVHDF has the advantages of CVVH and CVVHD and can achieve higher clearance efficiency by increasing replacement fluid or dialysate flow rate, which is conducive to the management of metabolic complications of RCA. Table 9 shows RCA applications in different CRRT modes.
RCA and energy management
Recommendation 16
In the energy management of patients with RCA-CRRT, the extra calories provided by this anticoagulant approach should be taken into account. However, due to the CRRT mode, treatment dose, different replacement fluids and different anticoagulant composition, the extra calories provided by RCA are difficult to accurately estimate. (Agreement degree: 95.2%, Evidence quality III, Recommendation strength C).
Remarks
Citrate is not only an intermediate product in the tricarboxylic acid cycle but also can be directly oxidized and decomposed. Complete oxidative decomposition of 1 mmol of citrate in the body can produce 0.59 kcal of heat [68, 69]. In addition, the additional glucose provided during anticoagulation with ACD-A also generates heat (complete oxidative decomposition of 1 mmol of glucose produces 0.73 kcal of heat). As shown in Table 10, the amount of energy substrate entering the body varies with the use of different citrate anticoagulant solutions and different replacement fluid or dialysate flow rates. Therefore, RCA can have an impact on patients’ caloric management. Anticoagulation alone can generate 350–900 kcal extra per day, which should not be ignored but be included in the overall nutritional management of patients. However, due to the CRRT mode, treatment dose, different replacement fluids and different anticoagulant composition, the extra calories provided by RCA are difficult to accurately estimate. In addition, the effect on energy management is more complex when different formulations of replacement fluid are used, such as lactate replacement fluid (complete oxidative decomposition of 1 mmol lactic acid produces 0.33 kcal) or high glucose replacement fluid [20, 70].
Summary
RCA has many advantages over SHA and is recommended as the preferred anticoagulant method for CRRT. However, if the parameters are not set properly or monitoring is not standardized, metabolic acid–base and electrolyte disturbances can still occur. Citrate accumulation is a rare and fatal complication of RCA, but the higher risk of death may be primarily due to severe comorbid diseases rather than citrate accumulation itself. There are no absolute contraindications to using RCA. Shock, severe liver dysfunction, or hyperlactatemia should not be considered as absolute contraindications to RCA. These patients should be considered as belonging to a higher-risk group that should be monitored closely during treatment. Some CRRT modes (e.g., SCUF) are not suitable for RCA. In addition, RCA delivers additional energy substrates, which should be incorporated into overall daily nutrition management. Of course, many issues in clinical practice are very individual, and this guideline does not cover all RCA-related issues. As experience accumulates and evidence is added, the recommendation statements will be updated accordingly.
Availability of data and materials
Not applicable.
Abbreviations
- ACD-A:
-
Acid-citrate-dextrose formula A
- ALT:
-
Alanine transaminase
- AST:
-
Aspartate transaminase
- CCC:
-
Citrate-calcium complexes
- CMDA:
-
Chinese medical doctor association
- CRRT:
-
Continuous renal replacement therapy
- CVVH:
-
Continuous veno-venous hemofiltration
- CVVHD:
-
Continuous veno-venous hemodialysis
- CVVHDF:
-
Continuous veno-venous hemodiafiltration
- GRADE:
-
Grading of recommendations assessment, development and evaluation
- HCT:
-
Hematocrit
- HIT:
-
Heparin induced thrombocytopenia
- iCa:
-
Ionized calcium
- KDIGO:
-
Kidney disease improving global outcomes
- MELD:
-
Model of end-stage liver disease
- RCA:
-
Regional citrate anticoagulation
- SCUF:
-
Slow continuous ultrafiltration
- SHA:
-
Systemic heparin anticoagulation
- SID:
-
Strong ion difference
- TSC:
-
Trisodium citrate
References
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179–84.
Moberg J, Oxman AD, Rosenbaum S, Schünemann HJ, Guyatt G, Flottorp S, et al. The GRADE evidence to decision (EtD) framework for health system and public health decisions. Health Res Policy Syst. 2018;16(1):45.
Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care. 2016;20(1):144.
Schilder L, Nurmohamed SA, Bosch FH, Purmer IM, den Boer SS, Kleppe CG, et al. Citrate anticoagulation versus systemic heparinisation in continuous venovenous hemofiltration in critically ill patients with acute kidney injury: a multi-center randomized clinical trial. Crit Care. 2014;18(4):472.
Kośka A, Kirwan CJ, Kowalik MM, Lango-Maziarz A, Szymanowicz W, Jagielak D, et al. Filter life span in postoperative cardiovascular surgery patients requiring continuous renal replacement therapy, using a postdilution regional citrate anticoagulation continuous hemofiltration circuit. Cardiol J. 2022;29(1):53–61.
Zarbock A, Küllmar M, Kindgen-Milles D, Wempe C, Gerss J, Brandenburger T, et al. Effect of regional citrate anticoagulation vs systemic heparin anticoagulation during continuous kidney replacement therapy on dialysis filter life span and mortality among critically ill patients with acute kidney injury: a randomized clinical trial. JAMA. 2020;324(16):1629–39.
Wu MY, Hsu YH, Bai CH, Lin YF, Wu CH, Tam KW. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy: a meta-analysis of randomized controlled trials. Am J Kidney Dis. 2012;59(6):810–8.
Morabito S, Pistolesi V, Tritapepe L, Zeppilli L, Polistena F, Strampelli E, et al. Regional citrate anticoagulation in cardiac surgery patients at high risk of bleeding: a continuous veno-venous hemofiltration protocol with a low concentration citrate solution. Crit Care. 2012;16(3):R111.
Kindgen-Milles D, Brandenburger T, Dimski T. Regional citrate anticoagulation for continuous renal replacement therapy. Curr Opin Crit Care. 2018;24(6):450–4.
Lee PZ. Citric acid metabolism in normal human. Tianjin Med J. 1985;9:571–3.
Oudemans-van Straaten HM, Bosman RJ, Koopmans M, van der Voort PH, Wester JP, van der Spoel JI, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med. 2009;37(2):545–52.
Sık G, Demirbuga A, Annayev A, Citak A. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement therapy in critically ill children. Int J Artif Organs. 2020;43(4):234–41.
Hetzel GR, Schmitz M, Wissing H, Ries W, Schott G, Heering PJ, et al. Regional citrate versus systemic heparin for anticoagulation in critically ill patients on continuous venovenous haemofiltration: a prospective randomized multicentre trial. Nephrol Dial Transplant. 2011;26(1):232–9.
Gattas DJ, Rajbhandari D, Bradford C, Buhr H, Lo S, Bellomo R. A randomized controlled trial of regional citrate versus regional heparin anticoagulation for continuous renal replacement therapy in critically ill adults. Crit Care Med. 2015;43(8):1622–9.
Li R, Gao X, Zhou T, Li Y, Wang J, Zhang P. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis of randomized controlled trials. Ther Apher Dial. 2022;26(6):1086–97.
Meersch M, Küllmar M, Wempe C, Kindgen-Milles D, Kluge S, Slowinski T, et al. Regional citrate versus systemic heparin anticoagulation for continuous renal replacement therapy in critically ill patients with acute kidney injury (RICH) trial: study protocol for a multicentre, randomised controlled trial. BMJ Open. 2019;9(1):e024411.
Morabito S, Pistolesi V, Tritapepe L, Fiaccadori E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol. 2014;9(12):2173–88.
Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus. 2009;2(6):439–47.
Karkar A, Ronco C. Prescription of CRRT: a pathway to optimize therapy. Ann Intensive Care. 2020;10(1):32.
Balik M, Zakharchenko M, Otahal M, Hruby J, Polak F, Rusinova K, et al. Quantification of systemic delivery of substrates for intermediate metabolism during citrate anticoagulation of continuous renal replacement therapy. Blood Purif. 2012;33(1–3):80–7.
Legrand M, Tolwani A. Anticoagulation strategies in continuous renal replacement therapy. Semin Dial. 2021;34(6):416–22.
Alvarez G, Chrusch C, Hulme T, Posadas-Calleja JG. Renal replacement therapy: a practical update. Can J Anaesth. 2019;66(5):593–604.
Honore PM, De Bels D, Preseau T, Redant S, Spapen HD. Citrate: how to get started and what, when, and how to monitor? J Transl Int Med. 2018;6(3):115–27.
Zheng LJ, Jiang W, Pan L, Pan J. Reduced serum albumin as a risk factor for poor prognosis in critically ill patients receiving renal replacement therapy. BMC Nephrol. 2021;22(1):305.
Szamosfalvi B, Puri V, Sohaney R, Wagner B, Riddle A, Dickinson S, et al. Regional citrate anticoagulation protocol for patients with presumed absent citrate metabolism. Kidney360. 2020;2(2):192–204.
Nalesso F, Cattarin L, Calò LA. Regional citrate anticoagulation dose for continuous renal replacement therapy. Nephrology (Carlton). 2020;25(4):361.
Bi X, Zhang Q, Zhuang F, Lu W, Ding F. Optimized calcium supplementation approach in post-dilution CVVHDF using regional citrate anticoagulation. Int J Artif Organs. 2021;44(3):165–73.
Yang NN, Lee ZS, Yang L, Chen WF. Correlation between ionic calcium after filter and in vivo during RCA-CRRT treatment. Modern Pract Med. 2021;33(8):993–5.
Zatorski P, Abokhouskaya N, Łącki P, Kołacz M, Trzebicki J. Ionized calcium measurements during continuous renal replacement therapy with regional citrate anticoagulation. Clin Chem Lab Med. 2021;59(3):e107–9.
Medrano C, Cointault O, Lavayssiere L, Nogier MB, Colliou E, Setbon N, et al. Heparin-free regional anticoagulation of haemodialysis filters with calcium-free dialysate: is citrate mandatory? Clin Kidney J. 2021;14(12):2534–8.
Khadzhynov D, Deissler J, Bobonov O, Schelter C, Peters H, Kindgen-Milles D, et al. Intensive monitoring of post filter ionized calcium concentrations during CVVHD with regional citrate anticoagulation: a retrospective study. J Crit Care. 2020;58:1–5.
Oudemans-van Straaten HM, Ostermann M. Citrate anticoagulation for CRRT: don’t always trust the postfilter iCa results! Crit Care. 2015;19:429.
Bos JC, Grooteman MP, van Houte AJ, Schoorl M, van Limbeek J, Nubé MJ. Low polymorphonuclear cell degranulation during citrate anticoagulation: a comparison between citrate and heparin dialysis. Nephrol Dial Transplant. 1997;12(7):1387–93.
Gritters M, Grooteman MP, Schoorl M, Schoorl M, Bartels PC, Scheffer PG, et al. Citrate anticoagulation abolishes degranulation of polymorphonuclear cells and platelets and reduces oxidative stress during haemodialysis. Nephrol Dial Transplant. 2006;21(1):153–9.
Orsag A, Bozic-Mijovski M, Hudoklin S, Simcic S, Gubensek J. Biocompatibility parameters with standard and increased dose of citrate in hemodialysis-a randomized trial. J Clin Med. 2021;10(13):2987.
Campo S, Lacquaniti A, Trombetta D, Smeriglio A, Monardo P. Immune system dysfunction and inflammation in hemodialysis patients: two sides of the same coin. J Clin Med. 2022;11(13):3759.
Rhee H, Berenger B, Mehta RL, Macedo E. Regional citrate anticoagulation for continuous kidney replacement therapy with calcium-containing solutions: a cohort study. Am J Kidney Dis. 2021;78(4):550-9.e1.
Zhang L, Liao Y, Xiang J, Qin W, Wu X, Tang Y, et al. Simplified regional citrate anticoagulation using a calcium-containing replacement solution for continuous venovenous hemofiltration. J Artif Organs. 2013;16(2):185–92.
Wei T, Tang X, Zhang L, Lin L, Li P, Wang F, et al. Calcium-containing versus calcium-free replacement solution in regional citrate anticoagulation for continuous renal replacement therapy: a randomized controlled trial. Chin Med J (Engl). 2022;135(20):2478–87.
Bianchi NA, Altarelli M, Eckert P, Schneider AG. Complications of regional citrate anticoagulation for continuous renal replacement therapy: an observational study. Blood Purif. 2020;49(5):567–75.
Köglberger P, Klein SJ, Lehner GF, Bellmann R, Peer A, Schwärzler D, et al. Low bicarbonate replacement fluid normalizes metabolic alkalosis during continuous veno-venous hemofiltration with regional citrate anticoagulation. Ann Intensive Care. 2021;11(1):62.
Schneider AG, Journois D, Rimmelé T. Complications of regional citrate anticoagulation: accumulation or overload? Crit Care. 2017;21(1):281.
Morgan TJ. The Stewart approach–one clinician’s perspective. Clin Biochem Rev. 2009;30(2):41–54.
Adrogué HJ, Tucker BM, Madias NE. Clinical approach to assessing acid-base status: physiological vs stewart. Adv Chronic Kidney Dis. 2022;29(4):343–54.
Adrogué HJ, Madias NE. Assessing acid-base status: physiologic versus physicochemical approach. Am J Kidney Dis. 2016;68(5):793–802.
Adrogué HJ, Awan AA, Madias NE. Sodium fate after sodium bicarbonate infusion: influence of altered acid-base status. Am J Nephrol. 2020;51(3):182–91.
Bunker JP, Bendixen HH, Murphy AJ. Hemodynamic effects of intravenously administered sodium citrate. N Engl J Med. 1962;266:372–7.
Bolan CD, Cecco SA, Wesley RA, Horne M, Yau YY, Remaley AT, et al. Controlled study of citrate effects and response to i.v. calcium administration during allogeneic peripheral blood progenitor cell donation. Transfusion. 2002;42(7):935–46.
Bauer E, Derfler K, Joukhadar C, Druml W. Citrate kinetics in patients receiving long-term hemodialysis therapy. Am J Kidney Dis. 2005;46(5):903–7.
Kramer L, Bauer E, Joukhadar C, Strobl W, Gendo A, Madl C, et al. Citrate pharmacokinetics and metabolism in cirrhotic and noncirrhotic critically ill patients. Crit Care Med. 2003;31(10):2450–5.
Anstey CM, Russell FD. Measurement of the concentration of citrate in human biofluids in patients undergoing continuous renal replacement therapy using regional citrate anticoagulation: application of a two-step enzymatic assay. Blood Purif. 2021;50(6):848–56.
Khadzhynov D, Schelter C, Lieker I, Mika A, Staeck O, Neumayer HH, et al. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J Crit Care. 2014;29(2):265–71.
Khadzhynov D, Dahlinger A, Schelter C, Peters H, Kindgen-Milles D, Budde K, et al. Hyperlactatemia, lactate kinetics and prediction of citrate accumulation in critically ill patients undergoing continuous renal replacement therapy with regional citrate anticoagulation. Crit Care Med. 2017;45(9):e941–6.
Hetzel GR, Taskaya G, Sucker C, Hennersdorf M, Grabensee B, Schmitz M. Citrate plasma levels in patients under regional anticoagulation in continuous venovenous hemofiltration. Am J Kidney Dis. 2006;48(5):806–11.
Meier-Kriesche HU, Gitomer J, Finkel K, DuBose T. Increased total to ionized calcium ratio during continuous venovenous hemodialysis with regional citrate anticoagulation. Crit Care Med. 2001;29(4):748–52.
Anstey CM, Venkatesh B. A comparison of the commonly used surrogate markers for citrate accumulation and toxicity during continuous renal replacement therapy with regional citrate anticoagulation. Blood Purif. 2022;51(12):997–1005.
Slowinski T, Morgera S, Joannidis M, Henneberg T, Stocker R, Helset E, et al. Safety and efficacy of regional citrate anticoagulation in continuous venovenous hemodialysis in the presence of liver failure: the liver citrate anticoagulation threshold (L-CAT) observational study. Crit Care. 2015;19:349.
Yu Y, Bai M, Wei Z, Zhao L, Li Y, Ma F, Sun S. Regional citrate anticoagulation versus low molecular weight heparin anticoagulation for continuous venovenous hemofiltration in patients with severe hypercalcemia: a retrospective cohort study. Ren Fail. 2020;42(1):748–58.
Fülöp T, Zsom L, Rodríguez RD, Chabrier-Rosello JO, Hamrahian M, Koch CA. Therapeutic hypernatremia management during continuous renal replacement therapy with elevated intracranial pressures and respiratory failure. Rev Endocr Metab Disord. 2019;20(1):65–75.
Zhang W, Bai M, Yu Y, Li L, Zhao L, Sun S, et al. Safety and efficacy of regional citrate anticoagulation for continuous renal replacement therapy in liver failure patients: a systematic review and meta-analysis. Crit Care. 2019;23(1):22.
Saner FH, Treckmann JW, Geis A, Lösch C, Witzke O, Canbay A, et al. Efficacy and safety of regional citrate anticoagulation in liver transplant patients requiring post-operative renal replacement therapy. Nephrol Dial Transplant. 2012;27(4):1651–7.
Ma Y, Chen F, Xu Y, Wang M, Zhou T, Lu J, et al. Safety and efficacy of regional citrate anticoagulation during plasma adsorption plus plasma exchange therapy for patients with acute-on-chronic liver failure: a pilot study. Blood Purif. 2019;48(3):223–32.
Lion RP, Tufan Pekkucuksen N, Srivaths P, Desai MS, Arikan AA. The safety and efficacy of regional citrate anticoagulation in albumin-assisted liver dialysis for extracorporeal liver support in pediatric patients. Blood Purif. 2019;47(1–3):23–7.
Schultheiß C, Saugel B, Phillip V, Thies P, Noe S, Mayr U, et al. Continuous venovenous hemodialysis with regional citrate anticoagulation in patients with liver failure: a prospective observational study. Crit Care. 2012;16(4):R162.
Peng B, Lu J, Guo H, Liu J, Li A. Regional citrate anticoagulation for replacement therapy in patients with liver failure: a systematic review and meta-analysis. Front Nutr. 2023;10:1031796.
Thanapongsatorn P, Chaijamorn W, Sirivongrangson P, Tachaboon S, Peerapornratana S, Lumlertgul N, et al. Citrate pharmacokinetics in critically ill liver failure patients receiving CRRT. Sci Rep. 2022;12(1):1815.
Alsabbagh MM, Ejaz AA, Purich DL, Ross EA. Regional citrate anticoagulation for slow continuous ultrafiltration: risk of severe metabolic alkalosis. Clin Kidney J. 2012;5(3):212–6.
Rogers AR, Jenkins B. Calorie provision from citrate anticoagulation in continuous renal replacement therapy in critical care. J Intensive Care Soc. 2021;22(3):183–6.
Jonckheer J, Van Hoorn A, Oshima T, De Waele E. Bioenergetic balance of continuous venovenous hemofiltration, a retrospective analysis. Nutrients. 2022;14(10):2112.
Wechselberger S, Compton F, Schilling J. Impact of continuous veno-venous hemodialysis with regional citrate anticoagulation on non-nutritional calorie balance in patients on the ICU-the NUTRI-DAY study. Nutrients. 2022;15(1):63.
Acknowledgements
Many thanks to Joseph Harold Walline, MD, for his valuable professional advice and language support. Harold Walline, MD, is an associate professor from the Department of Emergency Medicine, Penn State Health Milton S. Hershey, Medical Center and Penn State College of Medicine, Hershey, Pennsylvania, USA. We would also like to thank Professor Bin Du from Peking Union Medical College Hospital for his professional advice and guidance.
Experts of the consensus panel (Chinese surnames in alphabetical order): Xu-Feng Chen (the First Affiliated Hospital of Nanjing Medical University), Yu-Guo Chen (Qilu Hospital of Shandong University), Zhi Chen (Jiangxi Provincial People's Hospital), Qing-Li Dou (the Second Affiliated Hospital of Shenzhen University), Jian Guan (the First Hospital of Tsinghua University), Yao-Song Gui (Peking Union Medical College Hospital), Zhong-Wei Huang (the Affiliated Hospital and Medical School of Nantong University), Kui Jin (the First Affiliated Hospital of USTC), Xiao-Min Li (the First People’s Hospital of Lianyungang), Dan-Ping Liu (Shaanxi People’s Hospital), Shu-Yuan Liu (the Sixth Medical Center of Chinese PLA General Hospital), Jing-Jun Lv (Renmin Hospital of Wuhan University), Yong Liu (Shenzhen Hospital of Southern Medical University), Chuan-Yun Qian (the First Affiliated Hospital of Kunming Medical University), Yi Shan (the Sixth Medical Center of Chinese PLA General Hospital), Yan Shi (the Second People’s Hospital of Huai'an), Feng Sun (the First Affiliated Hospital of Nanjing Medical University), Ming Sun (the Affiliated Suqian Hospital of Xuzhou Medical University), Ding-Yu Tan (Northern Jiangsu People’s Hospital), Hai-Ying Wu (the First Affiliated Hospital of Kunming Medical University), Jian Xia (Zhongnan Hospital of Wuhan University), Tian-Yu Xin (the Sixth Medical Center of Chinese PLA General Hospital), Feng Xu (Qilu Hospital of Shandong University), Jun Xu (Peking Union Medical College Hospital), Sheng-Yong Xu (Peking Union Medical College Hospital), Tie Xu (the Affiliated Hospital of Xuzhou Medical University), Xian-Liang Yan (the Affiliated Hospital of Xuzhou Medical University), Jian-Zhong Yang (the First Affiliated Hospital of Xinjiang Medical University), Ting Yang (the First Affiliated Hospital of Kunming Medical University), Lu Yin (Peking University Shenzhen Hospital), Xue-Zhong Yu (Peking Union Medical College Hospital), Yong-Wu Yu (Beijing Chuiyangliu Hospital), Jin-Song Zhang (the First Affiliated Hospital of Nanjing Medical University), Mao Zhang (the Second Affiliated Hospital of Zhejiang University School of Medicine), Qiu-Bin Zhang (the Second Affiliated Hospital of Hainan Medical College), Wei Zhang (the First Affiliated Hospital of Kunming Medical University), Hong-Yu Zhao (Shengjing Hospital of China Medical University), Xiao-Dong Zhao (the Fourth Medical Center of Chinese PLA General Hospital), Dong-Hui Zheng (the Second People’s Hospital of Huai’an), Ping Zhou (Sichuan Provincial People’s Hospital), Bao-Feng Zhu (Nantong First People’s Hospital), Hua-Dong Zhu (Peking Union Medical College Hospital).
Consultant experts outside China (in no particular order): Shi-Qian Shen, Associate Professor of Anaesthesia, Physician Investigator & Assistant Anesthetist, Anesthesia, Critical Care and Pain Medicine, Mass General Research Institute & Harvard Medical School, Email: sshen2@mgh.harvard.edu. John Prowle, Senior Clinical Lecturer in Intensive Care Medicine within Prof. Rupert Pearse’s Critical Care and Perioperative Medicine Research Group, Honorary Consultant Physician in Intensive Care Medicine and Renal Medicine at the Royal London Hospital, Email: j.prowle@qmul.ac.uk. Martin Bellgardt, Consultant at Katholisches Klinikum Bochum, Email: martin.bellgardt@gmx.de.
Funding
Not applicable.
Author information
Authors and Affiliations
Consortia
Contributions
JX is the principal executive of this guideline. He has organized and chaired several expert seminars, proposed the consensus agenda and coordinated the work and task division of the panel members. SYL, JX, LY, SYX, TY, KJ, QBZ, FS, DYT, TYX, XZY, XDZ are the main discussants and core members of working group, and have done a lot of work on guideline framework construction and critical information evaluation. JX, SYL and TY are responsible for methodology. SYL, SYX and LY are the co-first writing author of the manuscript, and co-authored the manuscript with TY, KJ, QBZ, FS, DYT and TYX. SYL and LY are the secretaries of the consensus panel and other panelists participate in literature review, data collection, recommendations discussions, sub-panel discussions and suggestions. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Ethical approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
All authors declare that they have no competing interests.
Supplementary Information
Additional file 1: Table S1
. Composition and concentration of commercial bicarbonate replacement fluid in China
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/1.0/) applies to the data made available in this article, unless otherwise stated in a credit line to the data.
About this article
Cite this article
Liu, SY., Xu, SY., Yin, L. et al. Management of regional citrate anticoagulation for continuous renal replacement therapy: guideline recommendations from Chinese emergency medical doctor consensus. Military Med Res 10, 23 (2023). https://doi.org/10.1186/s40779-023-00457-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s40779-023-00457-9