Abstract
Acute kidney injury (AKI) is defined as an abrupt decrease in kidney function, with the most severe form requiring some method of renal replacement therapy (RRT). The use of RRT is required in 5-10% of critically ill patients who develop severe AKI. Renal replacement therapy can be provided as either intermittent hemodialysis or one of the various modes of continuous renal replacement therapy (CRRT), with CRRT potentially conferring an advantage with respect to renal recovery and dialysis independence. There is no difference in mortality when comparing low (< 25 mL·kg−1·hr−1) vs high (> 40 mL·kg−1·hr−1) RRT dosing. Continuous renal replacement therapy may be run in different modes of increasing complexity depending on a given patient’s clinical needs. Regional citrate anticoagulation is recommended as the therapy of choice for the majority of critically ill patients requiring CRRT.
Résumé
L’insuffisance rénale aiguë (IRA) se définit par une réduction subite de la fonction rénale, et sa forme la plus grave nécessite un type de traitement substitutif. Le recours à un traitement substitutif de l’insuffisance rénale est nécessaire chez 5-10 % des patients critiques qui souffrent d’une IRA grave. Le traitement substitutif de l’insuffisance rénale peut prendre la forme d’une hémodialyse intermittente ou de l’un des divers modes de traitement substitutif de l’insuffisance rénale en continu, ce second type de traitement conférant potentiellement un avantage en matière de récupération de la fonction rénale et d’indépendance de la dialyse. Aucune différence de mortalité n’a été observée en comparant un traitement substitutif de l’insuffisance rénale à faible dose d’ultrafiltration (< 25 mL·kg−1·h−1) vs à dose élevée (> 40 mL·kg−1·h−1). Le traitement substitutif de l’insuffisance rénale en continu peut être réalisé selon différents modes de complexité croissante en fonction des besoins cliniques d’un patient donné. Une anticoagulation régionale au citrate est recommandée comme traitement de choix pour la majorité des patients critiques nécessitant un traitement substitutif de l’insuffisance rénale en continu.
Similar content being viewed by others
This narrative review is intended for physicians involved in the care of critically ill patients who may require renal replacement therapy (RRT). It is intended for the clinician who may not have formal training in critical care or nephrology, nor advanced knowledge of modes of dialysis. Although the use of intermittent hemodialysis (IHD) is important for scenarios such as severe hyperkalemia and certain toxidromes (e.g., acetylsalicylic acid and lithium overdose), this paper will limit its discussion to continuous RRT (CRRT) modes. The main areas of focus will be: i) an epidemiologic review of acute kidney injury (AKI), ii) timing of RRT, iii) understanding the physical dialysis circuit, iv) modes of dialysis, v) effluent dosing, and vi) anticoagulation (and its complications).
Epidemiology
The treatment of critically ill patients is increasingly complex, particularly as the population ages and age-related co-morbidities become superimposed on critical illness. Furthermore, technologic and other pharmacologic advances have allowed these complex patients to be rescued. Indeed, advanced modes of life-support technology have enabled critical care physicians to manage diseases in ways unimaginable a generation ago.1,2,3 One such mode, RRT, has gained wide acceptance in supporting patients with isolated AKI, or as part of multi-organ system failure. Importantly, AKI is not a disease per se but rather a heterogeneous syndrome with numerous, often overlapping etiologies. Depending on the definition used, AKI affects up to 25% of intensive care unit (ICU) patients and has an associated mortality ranging between 15% and 60%.4,5,6,7,8,9
The International Acute Dialysis Quality Initiative (ADQI) group10 defined AKI as an abrupt decrease in kidney function, but is not limited to oliguria nor anuria. The ADQI group emphasizes that AKI is best viewed as a continuum of renal injury, the most severe of which requires some form of RRT. Indeed, as a syndrome, it may include patients with traditionally “normal” renal indices but functional impairment relative to physiologic demand.11 While there is broad consensus that more sensitive and specific biomarkers to diagnose AKI are needed, changes in serum creatinine and urine output still form the basis of all diagnostic criteria for AKI.9,10,12,13 The ADQI consensus document on AKI has been updated,14 and many large international series have provided a consistent picture—i.e., AKI is associated with decreased overall survival, and increasing severity of AKI leads to increased chances of death.15,16,17,18,19,20,21,22,23,24,25 Even mild, reversible AKI can increase mortality and the need for long-term dialysis.18,21,23,26,27,28 Furthermore, AKI increases the long-term risk of cardiovascular disease and chronic kidney disease.29,30,31,32,33,34 Based on these facts, the interest in identifying and preventing AKI is understandable.35,36,37,38,39
Sepsis-associated AKI deserves special mention since sepsis is the most important risk factor in determining the need for RRT.40 Bagshaw et al. reported an AKI incidence of 42% in their Australian cohort of septic patients.41 A ten-year retrospective study of AKI in septic patients found that the use of RRT was steadily increasing in all cohorts, but mortality was declining.42 Although outpatient dialysis is traditionally intermittent, ICU studies show that intermittent and continuous modes can be used effectively in the ICU population.43,44 Nevertheless, in North America, Europe, and Australia, continuous modes predominate.45,46,47 Despite there being no proven survival advantage when intermittent hemodialysis is compared with CRRT in critically ill patients, CRRT appears to confer an advantage with respect to hemodynamic stability and better control of fluid balance, renal recovery, and dialysis independence.43,44,48 A recent meta-analysis showed that among 26 identified studies,49 CRRT was associated with higher rates of renal recovery compared with IHD. Disadvantages of CRRT are limited mobility, the need for continuous anticoagulation, and significantly higher costs relative to IHD.50
Timing of RRT
Although AKI is common in critically ill patients, only 5-10% of patients go on to require some form of RRT.46,51 That said, the use of RRT is rapidly increasing,33,52,53 likely because of the aging population and growing complexity of patients admitted to the ICU. Sparse evidence exists to direct the clinician as to when to initiate RRT.54 Two recent trials have specifically tried to address the initiation time of RRT in critically ill patients: the ELAIN (Effect of early vs delayed initiation of renal replacement therapy) trial55 and the AKIKI (Initiation strategies for renal replacement therapy in the intensive care unit) trial.56 The ELAIN study was a single-centre randomized trial of 231 predominately post-surgical patients. Early RRT was defined as initialization within eight hours of diagnosis of KIDGO (Kidney disease: Improving Global Outcomes)9 stage 2; delayed RRT was defined as initiation of RRT within 12 hr of stage 3 AKI; the median difference in actual time was 21 hr. Early RRT resulted in an impressive decrease in mortality compared with delayed RRT (39.3% vs 53.6%, respectively; P = 0.03) and greater renal recovery (53.6% vs 38.7%, respectively; P = 0.02).
The AKIKI study was a multicentre trial with 620 mixed medical/surgical patients with a different definition of early (less than six hours of KDIGO [Kidney Disease: Improving Global Outcomes] stage 3) vs late AKI (traditional criteria to worsening AKI or complications). The median difference to RRT initiation was 57 hr. There was no difference in 60-day mortality in the early compared with late groups (48.5% vs 49.7%, respectively; P = 0.79). Perhaps more interesting is the actual RRT utilization, which was 98% for early RRT compared with 51% for delayed RRT. In other words, the delayed arm avoided unnecessary RRT. The resulting inference for a clinician is intriguing but speculative—i.e., were there many patients in the early cohort that could have also avoided RRT? Some authors57,58 have cautioned readers on the conclusions of these studies. Both studies had limitations that reduced the confidence in their conclusions, including implausible treatment effect (both trials were powered assuming a > 15% mortality reduction), low fragility index (small number of patients required to convert a trial from being statistically significant to not significant),59 and ELAIN being a single-centre study. A recent study, with none of these short-comings, showed no difference in mortality when comparing early initiation with delayed initiation of RRT in septic patients.60
With these results, it is still difficult to provide a recommendation on when to initiate RRT. Nevertheless, the clinician can be reassured that starting RRT earlier does not increase mortality, but at the cost of an increased number of unnecessary treatments. The ongoing STAART-AKI (ClinicalTrials.gov identifier: NCT02568722) trial should help inform physicians when to initiate renal replacement therapy
The physical aspects of RRT
Dedicated vascular access is needed for RRT and can be obtained with a purpose-designed dual lumen catheter placed in a central vein using the Seldinger technique. The veins used in order of preference, as per KDIGO guidelines, are the internal jugular, femoral, or subclavian.9 The subclavian vein is the third choice due to a higher risk of thrombosis and stenosis.61 A non-tunneled catheter with its tip placed in the superior vena cava is favoured for immediate, short-term use. Chronic use favours tunneled catheters to decrease the risk of infection; the distal tip is usually placed in the right atrium. The catheter lumens are labelled and colour coded as the “arterial” (red) blood in-flow line, which is the proximal/intake port, and the “venous” (blue) blood out-flow line for the distal/discharge port.62
Interdialytic lock solution is used to decrease the risk of catheter thrombosis and the risk of infection. Heparin (1,000 U·mL−1 to 10,000 U·mL−1), citrate (4-47%), and tissue plasminogen activator have all been used. Heparin and citrate are equivalent in maintaining patency.63 The effectiveness of citrate and antimicrobial lock solutions in decreasing the rate of catheter-related infections remains unclear and is the subject of ongoing investigation.64 In addition to the risk of bleeding from an inadvertent bolus of the locking solution with catheter access, all catheters will leak locking solution for the first 30 min with some systemic effects occurring for up to four hours. Heparin carries a further risk of heparin-induced thrombocytopenia, and citrate risks a metallic taste and perioral and/or digital paresthesia.65
Standard CRRT mechanical units have an extracorporeal circuit with a warmer hemofilter and dedicated pump for blood. They may also have additional pumps that are used for other fluids that vary depending on the therapy mode, such as pumps for replacement fluid and dialysate. Incorporated scales are used for gravimetric fluid balancing.66
Filters are composed of approximately 10,000 hollow fibres with diameters of approximately 200 µm and a membrane thickness of 20-50 µm.67 The membrane material can be made from either cellulose-derived or synthetic polymers. Optimal biocompatibility is important so as to prevent damage to red blood cells and contact activation of neutrophils and platelets, either directly or through the activation of the coagulation cascade and the complement system.68 For example, the AN-69 synthetic polyacrolonitrile membrane can cause an anaphylactoid reaction due to bradykinin accumulation in patients receiving angiotensin converting enzyme inhibitors.69
Membranes vary by the number and size of pores, which influences permeability and the movement of water for a given transmembrane pressure, referred to as flux. They also vary in the degree to which larger solutes, such as inflammatory mediators, are adsorbed. Filters are available with different surface areas and volumes. A filter with an extracorporeal volume of > 200 mL becomes important in instances where the filter clots before the blood can be returned to the patient.
Renal replacement modalities
Water and solutes pass from the blood through the semipermeable membrane during dialysis mainly by ultrafiltration, convection, and diffusion.
Ultrafiltration and convection involve movement of water across the membrane due to a pressure gradient. Ultrafiltration refers to the movement of plasma water while convection is the movement of solutes within the plasma water. Convection is sometimes called “solvent drag” (Fig. 1). Diffusion is the movement of solute driven by the concentration gradient across a semipermeable membrane. In dialysis, it is the gradient between the patient’s blood on one side of the filter and the dialysate on the other.
Running the dialysate counter-current to the blood increases the removal of small solutes such as urea and creatinine.70
Continuous RRT may be run in different modes of increasing complexity depending on a given patient’s clinical needs.66 A schematic of the different modalities is shown in Figs 2, 3, 4, 5 and Table 1. The modes differ in whether the primary driver of solute removal is convection, diffusion, or both.
Slow continuous ultrafiltration
Slow continuous ultrafiltration (SCUF) is used to remove plasma water in patients without significant electrolyte or other acid-base abnormalities. Blood is pumped through the fibres of the dialysis filter at a pressure higher than that surrounding the fibres. The hydrostatic pressure gradient between the blood compartment of the filter and the filtrate compartment is the transmembrane pressure, which determines the rate of fluid removal. Using higher flux membranes allows for more fluid removal at the same transmembrane pressure. While SCUF has the advantage of decreased complexity and nursing workload compared with other modes, it cannot correct electrolyte or acid-base abnormalities. While the major effect of SCUF is fluid removal, some solute clearance occurs because of convection, but at a much lower efficiency than other continuous modes described below.
Continuous veno-venous hemofiltration
Continuous veno-venous hemofiltration (CVVH) uses convection to remove solutes through large volume fluid ultrafiltration. Convection sweeps solutes along with the fluid independent of their concentration gradient. The porosity of the membrane determines which solutes are removed. Small solute molecules, such as urea, and middle-sized molecules, such as inflammatory cytokines, are cleared. With the large volume of fluid removed, intravascular volume must be maintained using a replacement fluid. The prescription is based on the patient’s serum potassium and acid-base balance. For example, bicarbonate containing fluids are used in the setting of a metabolic acidosis or normal saline when significant metabolic alkalosis develops. The replacement fluid can be infused either before the hemofilter (pre-dilution) or after the hemofilter (post-dilution). Post-dilution results in more concentrated blood in the filter and higher solute clearance. Nevertheless, more concentrated blood can lead to a shorter filter lifespan. While pre-dilution means lower solute concentrations and clearance, this is offset by a higher ultrafiltration rate and longer filter life. Pre-dilution does require a larger volume of replacement fluid than post-dilution.
Hemofiltration allows for volume removal as well as correction of electrolyte and acid-based abnormalities based on the selection of replacement fluid.
Continuous veno-venous hemodialysis
Continuous veno-venous hemodialysis (CVVHD) uses counter-current dialysate flow to remove small solutes by diffusion according to their concentration gradients. Solute clearance can be increased with higher dialysate or blood flow rates.
Dialysates contain physiologic concentrations of sodium, chloride, magnesium, and glucose. Serum potassium can vary significantly in critical illness depending on factors such as pH, insulin and sympathomimetic drugs, gastrointestinal losses, residual or recovering renal function, and high hemofiltration rates. The potassium concentration of the dialysate is prescribed separately usually ranging from 0 to 5 mmol·L−1. It is not unusual for the potassium prescription to change frequently. Dialysate is buffered with either bicarbonate or a bicarbonate precursor such as lactate, citrate, or acetate. The use of bicarbonate precursors requires the patient to be able to metabolize them, which can be impaired in liver failure or shock states. Importantly, in CVVHD, there is minimal ultrafiltration and therefore no significant fluid removal.
Continuous veno-venous hemodiafiltration
Continuous veno-venous hemodiafiltration combines hemodialysis (diffusive dialysis) and hemofiltration (convective dialysis). The ultrafiltrate can be replaced by either replacement fluid as in hemofiltration and the counter-current/co-current dialysate flow.
The choice of CRRT mode is determined by the patient’s volume status, serum urea, and potassium, as well as acid-base balance. Slow continuous ultrafiltration could be considered in conditions with isolated volume overload, such as heart or liver failure, malnutrition, capillary leak syndromes, or in patients who have become resistant to diuretics. Isolated electrolyte abnormalities can be managed with hemodialysis (CVVHD). Nevertheless, most critically ill patients receive large amounts of intravenous fluids as part of their resuscitation and ongoing prescriptions and nutrition. This means that those with kidney injuries usually require ongoing management with fluids and electrolytes. This can be accomplished using either hemofiltration (CVVH) using appropriate replacement fluid or with hemodiafiltration (CVVHDF) depending on the medical centre’s preference.
Effluent dosing
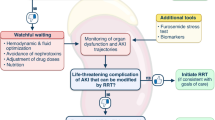
Similar to any drug, a physician prescribes the RRT “dose” based on a wide variety of pharmacokinetic and pharmacodynamic principles. Among many things, the clinician must take into account factors such as age, body weight, mode of elimination, and co-administered drugs. Dosing of RRT is traditionally defined as the effluent flow in mL·kg−1·hr−1. In particular, the dose intensity has been of great interest and studies have compared low (< 25 mL·kg−1·hr−1) vs high (> 40 mL·kg−1·hr−1)71,72 effluent rates. The RENAL (Intensity of continuous renal replacement therapy in critically ill patients) study provided the best evidence that there are no differences in patients treated with these two strategies.73 In that study, at 90 days after randomization, death occurred in 44.7% of patients for both higher and lower intensity groups (odds ratio, 1.00; 95% confidence interval, 0.81 to 1.23; P = 0.99). Furthermore, there were no significant differences between the groups in any of the secondary or tertiary outcomes. Fig. 1 outlines the expert consensus opinion to guide the clinician for best practice when dosing RRT.74 To ensure outcomes similar to those seen in the ATN and RENAL trials, this figure shows the recommendation that the clinician provide an average daily effluent dosing between 20 and 25 mL·kg−1·hr−1. No evidence to date suggests incremental benefit to higher effluent dosing.
A common problem in dosing studies is that the actual intended delivery dose often falls short of the prescribed dose.73,75 Claure-Del Granado et al. addressed this problem by physically measuring the urea clearance adjusting for machine down-time and pre-dilution.76 As mentioned earlier, pre-dilution is when the replacement fluid is mixed with the blood (thus lowering hematocrit) prior to the blood entering the filter. Although this increases filter life, less efficient clearance occurs vs post-filter replacement. These authors observed that the actual dose significantly underestimated the prescribed dose by nearly 25%. Interestingly, the authors observed that the major factors affecting the treatment time had little to do with filter clotting, as one might predict with an extracorporeal circuit that requires anticoagulation. Common interruptions to delivering RRT were surgical procedures, the decision when to transition to IHD, and line or machine malfunction. The authors concluded that to achieve a prescribed dialysis dose, effluent based dosing should be increased by 20-25% to account for decreases in treatment time and reduced filter efficacy during CRRT. The KDIGO guidelines endorse the recommendation to increase effluent dosing by 25% to achieve best practice as shown in Fig. 6.9
Anticoagulation
Extracorporeal membrane clotting is a major concern during RRT. Clot-related membrane dysfunction is associated with decreased solute clearance, particularly for middle molecular weight molecules (500 D to 60 kD) such as B2-microglobulin and light chain proteins.69,75,77,78 Therefore, effective anticoagulation is paramount to prevent clotting of the circuit and to optimize filter efficiency. There are two major anticoagulation strategies: systemic and regional (Table 2).
Systemic anticoagulation
Systemic anticoagulation with unfractionated heparin (UFH) is low cost, the activated partial thromboplastin time is easy to monitor, and uses protamine as a reversal agent; therefore, it is the most widely method used worldwide.78
The UFH is usually delivered to the patient by a separate intravenous line. Nevertheless, UFH use is associated with increased risk of bleeding (particularly in critically ill patients),79 heparin-induced thrombocytopenia (HIT),80 and potentially deleterious pro-inflammatory effects because it binds to the lysyl-residue of antithrombin and accelerates the interaction between thrombin and antithrombin, thereby inhibiting the anti-inflammatory action of antithrombin,81 and triggering the release of inflammatory mediators from endothelial cells.82
Direct thrombin inhibitors such as argatroban are commonly used as alternative anticoagulants for patients with HIT. Its use is associated with shorter filter patency time but fewer bleeding complications.83 Of note, regional RRT anticoagulation does not provide sufficient anticoagulation in the presence of HIT, and a combination of regional and systemic anticoagulation with a direct thrombin inhibitor is recommended.14
Finally, low molecular weight heparins act by binding predominantly to factor Xa; this effect is associated with fewer adverse events than UFH.84 Low molecular weight heparins are eliminated through the kidneys, which limits their use in patients requiring RRT. Furthermore, monitoring the anticoagulant effect requires a close range of anti-Xa activity. This is an expensive test that is not widely available and may take up to 24 hr to give results, which negatively impacts therapeutic decision making.85 There is insufficient evidence to support the use of low molecular weight heparins during CRRT and as such, it is not a recommended anticoagulation strategy.9
Regional anticoagulation
A practical alternative to systemic anticoagulation is regional extracorporeal citrate anticoagulation (RCA). Sodium citrate is infused into the proximal limb of the CRRT circuit where it chelates ionized calcium. The resulting complex is partially filtered across the CRRT membrane, which prevents clotting in the CRRT circuit. Hypocalcemia is prevented through an infusion of calcium with the blood returning to the patient.86
Demonstrable advantages of RCA include citrate’s short half-life, longer CRRT filter life spans, and a reduced occurrence of bleeding.80,87,88,89 Disadvantages of RCA are the patient’s inability to metabolize citrate (e.g., liver failure) within the systemic circulation, the complexity of citrate protocols, and citrate toxicity.9,90 Relative contraindications include patients with acute liver dysfunction or severe cirrhosis.90,91 When administered through the pre-filter line, citrate chelates calcium ions producing a soluble complex that is unavailable for calcium-dependent reactions in the intrinsic and extrinsic coagulation pathways.92 The target post-filter ionized calcium (iCa2+) concentration is between 0.26 and 0.35 mmol·L−1.93 It is preferable to use calcium-free dialysate or replacement fluids to minimize citrate requirements.94 The citrate infusion rate required depends on the concentration of the citrate solution and the blood flow in the circuit. Citrate that is not removed by the CRRT circuit enters the patient’s circulation and is normally metabolized in the liver, kidney, or skeletal muscle with no anticoagulant effect on the patient.
Several studies have reported the safety and efficacy of RCA compared with systemic anticoagulation during the different modalities of RRT.80,95,96,97 It is clear that RCA is associated with longer circuit survival time and reduced risk of bleeding.98,99 RCA can be recommended as the therapy of choice for the majority of critically ill patients requiring CRRT.9
Given that citrate is predominantly metabolized by the liver, there is a reduced mitochondrial citrate metabolism in patients with liver failure that results in citrate accumulation and consequently, secondary hypocalcemia and metabolic alkalosis.
Measurement of blood or plasma citrate is not readily available or timely.97,100,101 Thus, the total to ionized calcium ratio is a practical and specific marker of citrate accumulation (goal ratio < 2.3) and should be calculated at least every 12 hr.102,103 Clinical signs of citrate toxicity relative to ionized hypocalcemia include coagulopathy and cardiac toxicity such as prolonged QTc interval, decreased contractility, hypotension and cardiac arrest.100 Additionally, secondary to its nature as an ion and acting as a weak acid in solutions, citrate accumulation may produce a degree of anion gap metabolic acidosis; nevertheless, after citrate is metabolized by the liver, an excess of cations ensues and the result is metabolic alkalosis. Citrate anticoagulation has metabolic abnormalities unrelated to its impaired metabolism.87,91,100 The citrate chelates magnesium and crosses the CRRT filter, leading to hypomagnesemia. Tri-sodium citrate solutions are hypertonic due to their high sodium content. Low sodium replacement and dialysate solutions are used to prevent a patient developing hypernatremia.
There are several strategies to avoid citrate accumulation. These include ensuring that no added citrate is being used (e.g., with blood products administration). In addition, the citrate infusion rate can be decreased by 75% of the dose being used to that point. This is accompanied by an increase in the blood flow rate and replacement fluid and dialysate rate by at least 25%. A third option is to continue titrating the calcium chloride infusion rate, and lastly, if the ratio increases, the citrate infusion can be stopped and a non-anticoagulation RRT modality can be initiated.94 Safe implementation of citrate requires a well-designed and flexible protocol with adjustable dosing and monitoring; strict adherence to the protocol and its algorithm can prevent metabolic complications. It is feasible and safe to use RCA even in patients with liver failure.100
Conclusions
Acute kidney injury is a complex and frequent complication among critically ill patients. When AKI occurs, RRT is required in about one out of ten patients. Although there is no mortality difference between IHD and CRRT, the latter seems to provide better renal recovery and dialysis independence, and is the therapy of choice in hemodynamically unstable patients. CRRT can be provided in different modes of increasing complexity depending on a given patient’s clinical needs. Systemic anticoagulation with UFH is the most common strategy worldwide but RCA is currently the recommended therapy of choice in patients requiring CRRT.
References
Mitzner SR, Stange J, Klammt S, Peszynski P, Schmidt R, Noldge-Schomburg G. Extracorporeal detoxification using the molecular adsorbent recirculating system for critically ill patients with liver failure. J Am Soc Nephrol 2001; 12(Suppl 17): S75-82.
Beurtheret S, Mastroianni C, Pozzi M, et al. Extracorporeal membrane oxygenation for 2009 influenza A (H1N1) acute respiratory distress syndrome: single-centre experience with 1-year follow-up. Eur J Cardiothorac Surg 2012; 41: 691-5.
Zeiler FA, Matuszczak M, Teitelbaum J, Kazina CJ, Gillman LM. Plasmapheresis for refractory status epilepticus, part 1: a scoping systematic review of the adult literature. Seizure 2016; 43: 14-22.
Liano F, Pascual J. Epidemiology of acute renal failure: a prospective, multicenter, community-based study. Madrid Acute Renal Failure Study Group. Kidney Int 1996; 50: 811-8.
Brivet FG, Kleinknecht DJ, Loirat P, Landais PJ. Acute renal failure in intensive care units—causes, outcome, and prognostic factors of hospital mortality; a prospective, multicenter study. French Study Group on Acute Renal Failure. Crit Care Med 1996; 24: 192-8.
Uchino S, Kellum JA, Bellomo R, al. Acute renal failure in criticallly ill patients: a multinational, multicenter study. JAMA 2005; 294: 813-8.
Ratanarat R, Hantaweepant C, Tangkawattanakul N, Permpikul C. The clinical outcome of acute kidney injury in critically ill Thai patients stratified with RIFLE classification. J Med Assoc Thai 2009; 92(Suppl 2): S61-7.
Chen YC, Jenq CC, Tian YC, et al. RIFLE classification for predicting in-hospital mortality in critically ill sepsis patients. Shock 2009; 31: 139-45.
Kellum JA, Lameire N, Aspelin P, et al. KDIGO clinical practice guideline for acute kidney injury. Kidney International Supplements 2012; 2: 1-138.
Bellomo R, Ronco C, Kellum JA, Mehta RL, Palevsky P; Acute Dialysis Quality Initiative workgroup. Acute renal failure - definition, outcome measures, animal models, fluid therapy and information technology needs: the Second International Consensus Conference of the Acute Dialysis Quality Initiative (ADQI) Group. Crit Care 2004; 8: R204-12.
Eknoyan G, Lameire N, Barsoum R, et al. The burden of kidney disease: Improving global outcomes. Kidney Int 2004; 66: 1310-4.
Mehta R, Kellum JA, Shah SV, et al. Acute Kidney Injury Network: report of an initiative to improve outcomes in acute kidney injury. Crit Care 2007; 11: R31.
Ali T, Khan I, Simpson W, et al. Incidence and outcomes in acute kidney injury: a comprehensive population-based study. J Am Soc Nephrol 2007; 18: 1292-8.
Chawla LS, Bellomo R, Bihorac A, et al. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol 2017; 13: 241-57.
Abosaif NY, Tolba YA, Heap M, Russell J, El Nahas AM. The outcome of acute renal failure in the intensive care unit according to RIFLE: model application, sensitivity, and predictability. Am J Kidney Dis 2005; 46: 1038-48.
Kellum JA, Bellomo R, Ronco C. Classification of acute kidney injury using RIFLE: what’s the purpose? Crit Care Med 2007; 35: 1983-4.
Jenq CC, Tsai MH, Tian YC, et al. RIFLE classification can predict short-term prognosis in critically ill cirrhotic patients. Intensive Care Med 2007; 33: 1921-30.
Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med 2007; 35: 1837-43.
Maccariello E, Soares M, Valente C, et al. RIFLE classification in patients with acute kidney injury in need of renal replacement therapy. Intensive Care Med 2007; 33: 597-605.
Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: a systematic review. Kidney Int 2008; 73: 538-46.
Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant 2008; 23: 1203-10.
Thakar CV, Christianson A, Freyberg R, Almenoff P, Render ML. Incidence and outcomes of acute kidney injury in intensive care units: a veterans administration study. Crit Care Med 2009; 37: 2552-8.
Joannidis M, Metnitz B, Bauer P, et al. Acute kidney injury in critically ill patients classified by AKIN versus RIFLE using the SAPS 3 database. Intensive Care Med 2009; 35: 1692-702.
Schiffl H, Lang SM, Fischer R. Long-term outcomes of survivors of ICU acute kidney injury requiring renal replacement therapy: a 10-year prospective cohort study. Clin Kidney J 2012; 5: 297-302.
Sawhney S, Marks A, Fluck N, Levin A, Prescott G, Black C. Intermediate and long-term outcomes of survivors of acute kidney injury episodes: a large population-based cohort study. Am J Kidney Dis 2017; 69: 18-28.
Hoste EA, Clermont G, Kersten A, et al. RIFLE criteria for acute kidney injury are associated with hospital mortality in critically ill patients: a cohort analysis. Crit Care 2006; 10: R73.
Uchino S, Bellomo R, Goldsmith D, Bates S, Ronco C. An assessment of the RIFLE criteria for acute renal failure in hospitalized patients. Crit Care Med 2006; 34: 1913-7.
Pannu N, James M, Hemmelgarn B, Klarenbach S; Alberta Kidney Disease Network. Association between AKI, recovery of renal function, and long-term outcomes after hospital discharge. Clin J Am Soc Nephrol 2013; 8: 194-202.
Amdur RL, Chawla LS, Amodeo S, Kimmel PL, Palant CE. Outcomes following diagnosis of acute renal failure in U.S. veterans: focus on acute tubular necrosis. Kidney Int 2009; 76: 1089-97.
Coca SG, Yusuf B, Shlipak MG, Garg AX, Parikh CR. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis 2009; 53: 961-73.
Wald R, Quinn RR, Luo J, et al. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 2009; 302: 1179-85.
Triverio PA, Martin PY, Romand J, Pugin J, Perneger T, Saudan P. Long-term prognosis after acute kidney injury requiring renal replacement therapy. Nephrol Dial Transplant 2009; 24: 2186-9.
Wald R, McArthur E, Adhikari NK, et al. Changing incidence and outcomes following dialysis-requiring acute kidney injury among critically ill adults: a population-based cohort study. Am J Kidney Dis 2015; 65: 870-7.
Heung M, Steffick DE, Zivin K, et al. Acute kidney injury recovery pattern and subsequent risk of CKD: an analysis of veterans health administration data. Am J Kidney Dis 2016; 67: 742-52.
Reddy VG. Prevention of postoperative acute renal failure. J Postgrad Med 2002; 48: 64-70.
Harel Z, Chan CT. Predicting and preventing acute kidney injury after cardiac surgery. Curr Opin Nephrol Hypertens 2008; 17: 624-8.
Venkataraman R. Can we prevent acute kidney injury? Crit Care Med 2008; 36(4 Suppl): S166-71.
Stewart J, Findlay G, Smith N, Kelly K, Mason M. Adding insult to injury: a review of the care of patients who died in hospital with a primary diagnosis of acute kidney injury (acute renal failure). London, UK: National Confidential Enquiry into Patient Outcome and Death; 2009. Available from URL: https://www.ncepod.org.uk/2009report1/Downloads/AKI_report.pdf (accessed December 2018).
Itenov TS, Berthelsen RE, Jensen JU, et al. Predicting recovery from acute kidney injury in critically ill patients: development and validation of a prediction model. Crit Care Resusc 2018; 20: 54-60.
Oppert M, Engel C, Brunkhorst FM, et al. Acute renal failure in patients with severe sepsis and septic shock—a significant independant risk factor for mortality: results from the German Prevalence Study. Nephrol Dial Transplant 2008; 23: 904-9.
Bagshaw SM, George C, Bellomo R; ANZICS Database Management Committee. Early acute kidney injury and sepsis: a multicentre evaluation. Crit Care 2008; 12: R47.
Sakhuja A, Kumar G, Gupta S, Mittal T, Taneja A, Nanchal RS. Acute kidney injury requiring dialysis in severe sepsis. Am J Respir Crit Care Med 2015; 192: 951-7.
Bagshaw SM, Berthiaume LR, Delaney A, Bellomo R. Continuous versus intermittent renal replacement therapy for critically ill patients with acute kidney injury: a meta-analysis. Crit Care Med 2008; 36: 610-7.
Schneider AG, Bellomo R, Bagshaw SM, et al. Choice of renal replacement therapy modality and dialysis dependence after acute kidney injury: a systematic review and meta-analysis. Intensive Care Med 2013; 39: 987-97.
Prowle JR, Bellomo R. Continuous renal replacement therapy: recent advances and future research. Nat Rev Nephrol 2010; 6: 521-9.
Tolwani A. Continuous renal-replacement therapy for acute kidney injury. N Engl J Med 2013; 368: 1160-1.
Hoste EA, Bagshaw SM, Bellomo R, et al. Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 2015; 41: 1411-23.
Wald R, Shariff SZ, Adhikari NK, et al. The association between renal replacement therapy modality and long-term outcomes among critically ill adults with acute kidney injury: a retrospective cohort study. Crit Care Med 2014; 42: 868-77.
Schoenfelder T, Chen X, Bleß HH. Effects of continuous and intermittent renal replacement therapies among adult patients with acute kidney injury. GMS Health Technol Assess 2017; DOI: https://doi.org/10.3205/hta000127.
Manns B, Doig CJ, Lee H, et al. Cost of acute renal failure requiring dialysis in the intensive care unit: clinical and resource implications of renal recovery. Crit Care Med 2003; 31: 449-55.
Rewa O, Bagshaw SM. Acute kidney injury-epidemiology, outcomes and economics. Nat Rev Nephrol 2014; 10: 193-207.
Siddiqui NF, Coca SG, Devereaux PJ, et al. Secular trends in acute dialysis after elective major surgery—1995 to 2009. CMAJ 2012; 184: 1237-45.
Hsu RK, McCulloch CE, Dudley RA, Lo LJ, Hsu CY. Temporal changes in incidence of dialysis-requiring AKI. J Am Soc Nephrol 2013; 24: 37-42.
Palevsky PM. Indications and timing of renal replacement therapy in acute kidney injury. Crit Care Med 2008; 36(4 Suppl): S224-8.
Zarbock A, Kellum JA, Schmidt C, et al. Effect of early vs delayed initiation of renal replacement therapy on mortality in critically ill patients with acute kidney injury: the ELAIN randomized clinical trial. JAMA 2016; 315: 2190-9.
Gaudry S, Hajage D, Schortgen F, et al. Initiation strategies for renal-replacement therapy in the intensive care unit. N Engl J Med 2016; 375: 122-33.
Wierstra BT, Kadri S, Alomar S, Burbano X, Barrisford GW, Kao RL. The impact of “early” versus “late” initiation of renal replacement therapy in critical care patients with acute kidney injury: a systematic review and evidence synthesis. Crit Care 2016; 20: 122.
Bagshaw SM, Lamontagne F, Joannidis M, Wald R. When to start renal replacement therapy in critically ill patients with acute kidney injury: comment on AKIKI and ELAIN. Crit Care 2016; 20: 245.
Walsh M, Srinathan SK, McAuley DF, et al. The statistical significance of randomized controlled trial results is frequently fragile: a case for a Fragility Index. J Clin Epidemiol 2014; 67: 622-8.
Barbar SD, Clere-Jehl R, Bourredjem A, et al. Timing of renal-replacement therapy in patients with acute kidney injury and sepsis. N Engl J Med 2018; 379: 1431-42.
Schillinger F, Schillinger D, Montagnac R, Milcent T. Post catheterisation vein stenosis in haemodialysis: comparative angiographic study of 50 subclavian and 50 internal jugular accesses. Nephrol Dial Transplant 1991; 6: 722-4.
Santoro D, Benedetto F, Mondello P, et al. Vascular access for hemodialysis: current perspectives. Int J Nephrol Renovasc Dis 2014; 7: 281-94.
Abdel Azim AB, El Said TW, El Said HW, et al. A randomized controlled clinical trial of 4% sodium citrate versus heparin as locking solution for temporary dialysis catheters among hemodialysis patients. Clin Nephrol 2018; 90: 341-9.
Arechabala MC, Catoni MI, Claro JC, et al. Antimicrobial lock solutions for preventing catheter-related infections in haemodialysis. Cochrane Database Syst Rev 2018; 4: CD010597.
Mokrzycki MH, Lok CE. Traditional and non-traditional strategies to optimize catheter function: go with more flow. Kidney Int 2010; 78: 1218-31.
Villa G, Neri M, Bellomo R, et al. Nomenclature for renal replacement therapy and blood purification techniques in critically ill patients: practical applications. Crit Care 2016; 20: 283.
Sakai K. Dialysis membranes for blood purification. Front Med Biol Eng 2000; 10: 117-29.
Kokubo K, Kurihara Y, Kobayashi K, Tsukao H, Kobayashi H. Evaluation of the biocompatibility of dialysis membranes. Blood Purif 2015; 40: 293-7.
Tielemans C, Madhoun P, Lenaers M, Schandene L, Goldman M, Vanherweghem JL. Anaphylactoid reactions during hemodialysis on AN69 membranes in patients receiving ACE inhibitors. Kidney Int 1990; 38: 982-4.
Baldwin I, Baldwin M, Fealy N, et al. Con-current versus counter-current dialysate flow during CVVHD. A Comparative study for creatinine and urea removal. Blood Purif 2016; 41: 171-6.
Ronco C, Bellomo R, Homel P, et al. Effects of different doses in continuous veno-venous haemofiltration on outcomes of acute renal failure: a prospective randomised trial. Lancet 2000; 356: 26-30.
Bouman CS, Oudemans-Van Straaten HM, Tijssen JG, Zandstra DF, Kesecioglu J. Effects of early high-volume continuous venovenous hemofiltration on survival and recovery of renal function in intensive care patients with acute renal failure: a prospective, randomized trial. Crit Care Med 2002; 30: 2205-11.
RENAL Replacement Therapy Study Investigators; Bellomo R, Cass A, Cole L, et al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627-38.
Prowle JR, Schneider A, Bellomo R. Clinical review: Optimal dose of continuous renal replacement therapy in acute kidney injury. Crit Care 2011; 15: 207.
Tolwani AJ, Campbell RC, Stofan BS, Lai KR, Oster RA, Wille KM. Standard versus high-dose CVVHDF for ICU-related acute renal failure. J Am Soc Nephrol 2008; 19: 1233-8.
Claure-Del Granado R, Macedo E, Chertow GM, et al. Effluent volume in continuous renal replacement therapy overestimates the delivered dose of dialysis. Clin J Am Soc Nephrol 2011; 6: 467-75.
Pasko DA, Churchwell MD, Salama NN, Mueller BA. Longitudinal hemodiafilter performance in modeled continuous renal replacement therapy. Blood Purif 2011; 32: 82-8.
Brandenburger T, Dimski T, Slowinski T, Kindgen-Milles D. Renal replacement therapy and anticoagulation. Best Pract Res Clin Anaesthesiol 2017; 31: 387-401.
Claure-Del Granado R, Macedo E, Soroko S, et al. Anticoagulation, delivered dose and outcomes in CRRT: the program to improve care in acute renal disease (PICARD). Hemodial Int 2014; 18: 641-9.
Oudemans-van Straaten HM, Kellum JA, Bellomo R. Clinical review: Anticoagulation for continuous renal replacement therapy—heparin or citrate? Crit Care 2011; 15: 202.
Rosenberg RD. Heparin action. Circulation 1974; 49: 603-5.
Warren BL, Eid A, Singer P, et al. Caring for the critically ill patient. High-dose antithrombin III in severe sepsis: a randomized controlled trial. JAMA 2001; 286: 1869-78.
Link A, Girndt M, Selejan S, Mathes A, Bohm M, Rensing H. Argatroban for anticoagulation in continuous renal replacement therapy. Crit Care Med 2009; 37: 105-10.
Thrombosis Canada: Dedicated To Furthering Education & Research in Thrombotic Disease. Thrombose Canada - Whitby, ON - 2018. Available from URL: https://thrombosiscanada.ca (accessed December 2018).
Joannidis M, Kountchev J, Rauchenzauner M, et al. Enoxaparin vs. unfractionated heparin for anticoagulation during continuous veno-venous hemofiltration: a randomized controlled crossover study. Intensive Care Med 2007; 33: 1571-9.
Liu C, Mao Z, Kang H, Hu J, Zhou F. Regional citrate versus heparin anticoagulation for continuous renal replacement therapy in critically ill patients: a meta-analysis with trial sequential analysis of randomized controlled trials. Crit Care 2016; 20: 144.
Joannidis M, Oudemans-van Straaten HM. Clinical review: Patency of the circuit in continuous renal replacement therapy. Crit Care 2007; 11: 218.
Khadzhynov D, Dahlinger A, Schelter C, et al. Hyperlactatemia, lactate kinetics and prediction of citrate accumulation in critically ill patients undergoing continuous renal replacement therapy with regional citrate anticoagulation. Crit Care Med 2017; 45: e941-6.
Bai M, Zhou M, He L, et al. Citrate versus heparin anticoagulation for continuous renal replacement therapy: an updated meta-analysis of RCTs. Intensive Care Med 2015; 41: 2098-110.
Bagshaw SM, Darmon M, Ostermann M, et al. Current state of the art for renal replacement therapy in critically ill patients with acute kidney injury. Intensive Care Med 2017; 43: 841-54.
Davenport A, Tolwani A. Citrate anticoagulation for continuous renal replacement therapy (CRRT) in patients with acute kidney injury admitted to the intensive care unit. NDT Plus 2009; 2: 439-47.
Bagshaw SM, Laupland KB, Boiteau PJ, Godinez-Luna T. Is regional citrate superior to systemic heparin anticoagulation for continuous renal replacement therapy? A prospective observational study in an adult regional critical care system. J Crit Care 2005; 20: 155-61.
Calatzis A, Toepfer M, Schramm W, Spannagl M, Schiffl H. Citrate anticoagulation for extracorporeal circuits: effects on whole blood coagulation activation and clot formation. Nephron 2001; 89: 233-6.
Oudemans-van Straaten HM, Ostermann M. Bench-to-bedside review: Citrate for continuous renal replacement therapy, from science to practice. Crit Care 2012; 16: 249.
Oudemans-van Straaten HM, Bosman RJ, Koopmans M, et al. Citrate anticoagulation for continuous venovenous hemofiltration. Crit Care Med 2009; 37: 545-52.
Durao MS, Monte JC, Batista MC, et al. The use of regional citrate anticoagulation for continuous venovenous hemodiafiltration in acute kidney injury. Crit Care Med 2008; 36: 3024-9.
Morabito S, Pistolesi V, Tritapepe L, Fiaccadori E. Regional citrate anticoagulation for RRTs in critically ill patients with AKI. Clin J Am Soc Nephrol 2014; 9: 2173-88.
Zhang Z, Hongying N. Efficacy and safety of regional citrate anticoagulation in critically ill patients undergoing continuous renal replacement therapy. Intensive Care Med 2012; 38: 20-8.
Kirwan CJ, Jackson L, Prowle JR. A continuous renal replacement therapy protocol on the updated Nikkiso Aquarius Platform using regional citrate as first-line anticoagulation significantly improves filter life span but the position of the vascular access is key. Blood Purif 2018; 45: 129-30.
Khadzhynov D, Schelter C, Lieker I, et al. Incidence and outcome of metabolic disarrangements consistent with citrate accumulation in critically ill patients undergoing continuous venovenous hemodialysis with regional citrate anticoagulation. J Crit Care 2014; 29: 265-71.
Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci 2017; 56: 28-30.
Link A, Klingele M, Speer T, et al. Total-to-ionized calcium ratio predicts mortality in continuous renal replacement therapy with citrate anticoagulation in critically ill patients. Crit Care 2012; 16: R97.
Bakker AJ, Boerma EC, Keidel H, Kingma P, van der Voort PH. Detection of citrate overdose in critically ill patients on citrate-anticoagulated venovenous haemofiltration: use of ionised and total/ionised calcium. Clin Chem Lab Med 2006; 44: 962-6.
Conflict of interest
George Alvarez, Carla Chrusch, Terry Hulme, and Juan G. Posadas-Calleja have no conflicts of interest or disclosures.
Editorial responsibility
This submission was handled by Dr. Hilary P. Grocott, Editor-in-Chief, Canadian Journal of Anesthesia.
Author contributions
All authors reviewed the relevant literature and wrote the manuscript. All authors participated in the revision process.
Funding
Dr. Alvarez has received educational grants through Baxter Gambro Canada.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Alvarez, G., Chrusch, C., Hulme, T. et al. Renal replacement therapy: a practical update. Can J Anesth/J Can Anesth 66, 593–604 (2019). https://doi.org/10.1007/s12630-019-01306-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-019-01306-x